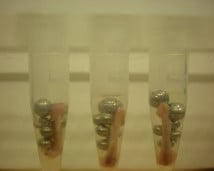
Publications where the Bullet Blender® was used to process samples from plants, algae or fungi, both single-celled and multicellular.
Plants
2474232
plants
1
apa
50
date
desc
1
3227
https://www.nextadvance.com/wp-content/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22LKCT9NIY%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Nguyen%20et%20al.%22%2C%22parsedDate%22%3A%222020-01-10%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ENguyen%2C%20H.%20M.%2C%20Yadav%2C%20N.%20S.%2C%20Barak%2C%20S.%2C%20Lima%2C%20F.%20P.%2C%20Sapir%2C%20Y.%2C%20%26amp%3B%20Winters%2C%20G.%20%282020%29.%20Responses%20of%20Invasive%20and%20Native%20Populations%20of%20the%20Seagrass%20Halophila%20stipulacea%20to%20Simulated%20Climate%20Change.%20%3Ci%3EFrontiers%20in%20Marine%20Science%3C%5C%2Fi%3E%2C%20%3Ci%3E6%3C%5C%2Fi%3E%2C%20812.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3389%5C%2Ffmars.2019.00812%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3389%5C%2Ffmars.2019.00812%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Responses%20of%20Invasive%20and%20Native%20Populations%20of%20the%20Seagrass%20Halophila%20stipulacea%20to%20Simulated%20Climate%20Change%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hung%20Manh%22%2C%22lastName%22%3A%22Nguyen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Narendra%20Singh%22%2C%22lastName%22%3A%22Yadav%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Simon%22%2C%22lastName%22%3A%22Barak%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fernando%20P.%22%2C%22lastName%22%3A%22Lima%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yuval%22%2C%22lastName%22%3A%22Sapir%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gidon%22%2C%22lastName%22%3A%22Winters%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222020-1-10%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.3389%5C%2Ffmars.2019.00812%22%2C%22ISSN%22%3A%222296-7745%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.frontiersin.org%5C%2Farticle%5C%2F10.3389%5C%2Ffmars.2019.00812%5C%2Ffull%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222020-01-24T18%3A06%3A14Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Chlorophyll%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Protein%22%7D%2C%7B%22tag%22%3A%22leaf%22%7D%2C%7B%22tag%22%3A%22leaves%22%7D%5D%7D%7D%2C%7B%22key%22%3A%2299NHABT6%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Diamos%20et%20al.%22%2C%22parsedDate%22%3A%222016-02-24%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EDiamos%2C%20A.%20G.%2C%20Rosenthal%2C%20S.%20H.%2C%20%26amp%3B%20Mason%2C%20H.%20S.%20%282016%29.%205%26%23x2032%3B%20and%203%26%23x2032%3B%20Untranslated%20Regions%20Strongly%20Enhance%20Performance%20of%20Geminiviral%20Replicons%20in%20Nicotiana%20benthamiana%20Leaves.%20%3Ci%3EFrontiers%20in%20Plant%20Science%3C%5C%2Fi%3E%2C%20%3Ci%3E7%3C%5C%2Fi%3E.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3389%5C%2Ffpls.2016.00200%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3389%5C%2Ffpls.2016.00200%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%225%5Cu2032%20and%203%5Cu2032%20Untranslated%20Regions%20Strongly%20Enhance%20Performance%20of%20Geminiviral%20Replicons%20in%20Nicotiana%20benthamiana%20Leaves%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrew%20G.%22%2C%22lastName%22%3A%22Diamos%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sun%20H.%22%2C%22lastName%22%3A%22Rosenthal%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hugh%20S.%22%2C%22lastName%22%3A%22Mason%22%7D%5D%2C%22abstractNote%22%3A%22We%20previously%20reported%20a%20recombinant%20protein%20production%20system%20based%20on%20a%20geminivirus%20replicon%20that%20yields%20high%20levels%20of%20vaccine%20antigens%20and%20monoclonal%20antibodies%20in%20plants.%20The%20bean%20yellow%20dwarf%20virus%20%28BeYDV%29%20replicon%20generates%20massive%20amounts%20of%20DNA%20copies%2C%20which%20engage%20the%20plant%20transcription%20machinery.%20However%2C%20we%20noticed%20a%20disparity%20between%20transcript%20level%20and%20protein%20production%2C%20suggesting%20that%20mRNAs%20could%20be%20more%20efficiently%20utilized.%20In%20this%20study%2C%20we%20systematically%20evaluated%20genetic%20elements%20from%20human%2C%20viral%2C%20and%20plant%20sources%20for%20their%20potential%20to%20improve%20the%20BeYDV%20system.%20The%20tobacco%20extensin%20terminator%20enhanced%20transcript%20accumulation%20and%20protein%20production%20compared%20to%20other%20commonly%20used%20terminators%2C%20indicating%20that%20efficient%20transcript%20processing%20plays%20an%20important%20role%20in%20recombinant%20protein%20production.%20Evaluation%20of%20human-derived%205%5Cu2032%20untranslated%20regions%20%28UTRs%29%20indicated%20that%20many%20provided%20high%20levels%20of%20protein%20production%2C%20supporting%20their%20cross-kingdom%20function.%20Among%20the%20viral%205%5Cu2032%20UTRs%20tested%2C%20we%20found%20the%20greatest%20enhancement%20with%20the%20tobacco%20mosaic%20virus%20omega%20leader.%20An%20analysis%20of%20the%205%5Cu2032%20UTRs%20from%20the%20Arabidopsis%20thaliana%20and%20Nicotinana%20benthamiana%20photosystem%20I%20K%20genes%20found%20that%20they%20were%20highly%20active%20when%20truncated%20to%20include%20only%20the%20near%20upstream%20region%2C%20providing%20a%20dramatic%20enhancement%20of%20transgene%20production%20that%20exceeded%20that%20of%20the%20tobacco%20mosaic%20virus%20omega%20leader.%20The%20tobacco%20Rb7%20matrix%20attachment%20region%20inserted%20downstream%20from%20the%20gene%20of%20interest%20provided%20significant%20enhancement%2C%20which%20was%20correlated%20with%20a%20reduction%20in%20plant%20cell%20death.%20Evaluation%20of%20Agrobacterium%20strains%20found%20that%20EHA105%20enhanced%20protein%20production%20and%20reduced%20cell%20death%20compared%20to%20LBA4301%20and%20GV3101.%20We%20used%20these%20improvements%20to%20produce%20Norwalk%20virus%20capsid%20protein%20at%20%3E20%25%20total%20soluble%20protein%2C%20corresponding%20to%201.8%20mg%5C%2Fg%20leaf%20fresh%20weight%2C%20more%20than%20twice%20the%20highest%20level%20ever%20reported%20in%20a%20plant%20system.%20We%20also%20produced%20the%20monoclonal%20antibody%20rituximab%20at%201%20mg%5C%2Fg%20leaf%20fresh%20weight.%22%2C%22date%22%3A%222016-2-24%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.3389%5C%2Ffpls.2016.00200%22%2C%22ISSN%22%3A%221664-462X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpmc%5C%2Farticles%5C%2FPMC4764687%5C%2F%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-06-10T19%3A12%3A42Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Leaves%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%2C%7B%22tag%22%3A%22Tobacco%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22UMX4VBPQ%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Riga%20et%20al.%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ERiga%2C%20P.%2C%20Benedicto%2C%20L.%2C%20Garc%26%23xED%3Ba-Flores%2C%20L.%2C%20Villa%26%23xF1%3Bo%2C%20D.%2C%20Medina%2C%20S.%2C%20%26amp%3B%20Gil-Izquierdo%2C%20%26%23xC1%3B.%20%282016%29.%20Rootstock%20effect%20on%20serotonin%20and%20nutritional%20quality%20of%20tomatoes%20produced%20under%20low%20temperature%20and%20light%20conditions.%20%3Ci%3EJournal%20of%20Food%20Composition%20and%20Analysis%3C%5C%2Fi%3E%2C%20%3Ci%3E46%3C%5C%2Fi%3E%2C%2050%26%23x2013%3B59.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.jfca.2015.11.003%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.jfca.2015.11.003%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Rootstock%20effect%20on%20serotonin%20and%20nutritional%20quality%20of%20tomatoes%20produced%20under%20low%20temperature%20and%20light%20conditions%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22Riga%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Leyre%22%2C%22lastName%22%3A%22Benedicto%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Libia%22%2C%22lastName%22%3A%22Garc%5Cu00eda-Flores%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22D%5Cu00e9bora%22%2C%22lastName%22%3A%22Villa%5Cu00f1o%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sonia%22%2C%22lastName%22%3A%22Medina%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22%5Cu00c1ngel%22%2C%22lastName%22%3A%22Gil-Izquierdo%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2203%5C%2F2016%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.jfca.2015.11.003%22%2C%22ISSN%22%3A%2208891575%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS088915751500246X%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-12-30T15%3A47%3A08Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Fruit%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Small%20molecules%22%7D%2C%7B%22tag%22%3A%22Tomato%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22J4C5MZKU%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Davis%20et%20al.%22%2C%22parsedDate%22%3A%222015-09-30%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EDavis%2C%20T.%20S.%2C%20Bosque-P%26%23xE9%3Brez%2C%20N.%20A.%2C%20Popova%2C%20I.%2C%20%26amp%3B%20Eigenbrode%2C%20S.%20D.%20%282015%29.%20Evidence%20for%20additive%20effects%20of%20virus%20infection%20and%20water%20availability%20on%20phytohormone%20induction%20in%20a%20staple%20crop.%20%3Ci%3EFrontiers%20in%20Ecology%20and%20Evolution%3C%5C%2Fi%3E%2C%20%3Ci%3E3%3C%5C%2Fi%3E.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3389%5C%2Ffevo.2015.00114%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3389%5C%2Ffevo.2015.00114%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Evidence%20for%20additive%20effects%20of%20virus%20infection%20and%20water%20availability%20on%20phytohormone%20induction%20in%20a%20staple%20crop%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%20S.%22%2C%22lastName%22%3A%22Davis%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nilsa%20A.%22%2C%22lastName%22%3A%22Bosque-P%5Cu00e9rez%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ina%22%2C%22lastName%22%3A%22Popova%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sanford%20D.%22%2C%22lastName%22%3A%22Eigenbrode%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222015-09-30%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.3389%5C%2Ffevo.2015.00114%22%2C%22ISSN%22%3A%222296-701X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fjournal.frontiersin.org%5C%2FArticle%5C%2F10.3389%5C%2Ffevo.2015.00114%5C%2Fabstract%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-12-30T20%3A31%3A03Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Leaves%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Small%20molecules%22%7D%2C%7B%22tag%22%3A%22Wheat%22%7D%5D%7D%7D%2C%7B%22key%22%3A%228I26UEGI%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mohammad%22%2C%22parsedDate%22%3A%222015-04-15%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EMohammad%2C%20A.-I.%20A.%20%282015%29.%20Electrophoretic%20analysis%20of%20proteins%20from%20different%20date%26%23xA0%3B%20palm%26%23xA0%3B%20%28Phoenix%20dactylifera%20L.%29%20cultivars%20in%20Saudi%20Arabia.%20%3Ci%3EAfrican%20Journal%20of%20Biotechnology%3C%5C%2Fi%3E%2C%20%3Ci%3E14%3C%5C%2Fi%3E%2815%29%2C%201325%26%23x2013%3B1333.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.5897%5C%2FAJB2014.14398%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.5897%5C%2FAJB2014.14398%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Electrophoretic%20analysis%20of%20proteins%20from%20different%20date%20%20palm%20%20%28Phoenix%20dactylifera%20L.%29%20cultivars%20in%20Saudi%20Arabia%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Al%20-%20Issa%20Adil%22%2C%22lastName%22%3A%22Mohammad%22%7D%5D%2C%22abstractNote%22%3A%22Fifteen%20%5Cn%2815%29%20%5Cnsamples%20of%20different%20date%20palm%20cultivars%20were%20collected%20from%20different%20locations%20in%20Al%5Cn-%5CnAhsa%20%5Cnoasis%20in%20the%20eastern%20province%20of%20Saudi%20Arabia.%20Extracted%20proteins%20from%20these%20samples%20were%20analyzed%20by%20%5Cnelectrophoresis%2C%20and%20clustered%20according%20to%20the%20average%20linkage%20between%20groups%20hierarchical%20clustering%20%5Cnmethod.%20The%20results%20reveal%20high%20degree%20of%20simi%5Cnlarity%20based%20on%20Jaccard%60s%20similarity%20method%20on%20the%20basis%20%5Cnof%20%20presence%20%20and%20%20absence%20%20of%20%20bands%2C%20%20that%20%20ranged%20%20between%20%200.421%20%20to%20%200.917%2C%20%20which%20%20was%20%20represented%20%20by%20%5Cnphylogenetic%20%20dendrogram%20%20in%20%20six%20%20clusters.%20%20The%20%20closely%20%20related%20%20cultivars%20%20%5C%22Hel%5C%22%20%20and%20%20%5C%22Hat%5C%22%20%20in%20%20addition%20%20to%20%5Cn%5C%22K%5Cnhl%5C%22%20represent%20the%20sixth%20cluster%2C%20which%20is%20separated%20out%20of%20other%20cultivars%20with%20high%20degree%20of%20similarity%20%5Cnthat%20ranged%20between%200.636%20to%200.714%3B%20it%20was%20confirmed%20by%20the%20first%20principal%20component%20with%20high%20loading%20%5Cn%2852.3%25%29%2C%20%20and%20%20characterized%20%20by%20%20four%20%20bands%20%20%2892%2C%20%5Cn100%2C%20%20205%20%20and%20%20108%20%20kda%29.%20%20These%20%20bands%20%20were%20%20mostly%20%5Cnpositioned%20close%20to%20each%20other%20in%20the%20scatter%20diagram.%20The%20second%20principal%20component%20with%20loading%20of%20%5Cn15.7%25%2C%20which%20were%20represented%20by%20three%20bands%2019%2C%2025%2C%20and%2037%20kda%2C%20have%20been%20confirmed%20the%20first%20cluster%20%5Cnof%20%20c%5Cnlosely%20%20related%20%20cultivars%20%20%5C%22Shi%5C%22%20%20and%20%20%5C%22Shl%5C%22%2C%20%20as%20%20well%20%20as%20%20the%20%20closely%20%20related%20%20cultivars%20%20%5C%22Mj%5C%22%20%20and%20%20%5C%22OmR%5C%22%20%5Cnamong%20%20the%20%20second%20%20cluster.%20%20It%20%20can%20%20be%20%20concluded%20%20that%20%20most%20%20of%20%20Al%5Cn-%5CnAhsa%20%20oasis%20%20date%20%20palm%20%20cultivars%20%20were%20%5Cnfrom%20%20one%20%20genetic%20%20origin%2C%20%20however%2C%20%20each%20%20cultivar%20%20was%20%20grown%20%20fr%5Cnom%20%20seed%20%20of%20%20locally%20%20known%20%20cultivar%2C%20%20and%20%5Cnlater%20was%20selected%20due%20to%20preferred%20fruit%20characteristics.%20More%20biochemical%20and%20molecular%20studies%20would%20%5Cnbe%20necessary%20to%20uncover%20the%20genetic%20relationships%20between%20area%20cultivars.%22%2C%22date%22%3A%2215%20April%202015%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.5897%5C%2FAJB2014.14398%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.ajol.info%5C%2Findex.php%5C%2Fajb%5C%2Farticle%5C%2Fview%5C%2F116747%5C%2F106323%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-08-06T15%3A52%3A33Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Date%20palm%22%7D%2C%7B%22tag%22%3A%22Leaves%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22KBBHB5CG%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Tierno%20et%20al.%22%2C%22parsedDate%22%3A%222015%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ETierno%2C%20R.%2C%20L%26%23xF3%3Bpez%2C%20A.%2C%20Riga%2C%20P.%2C%20Arazuri%2C%20S.%2C%20Jar%26%23xE9%3Bn%2C%20C.%2C%20Benedicto%2C%20L.%2C%20%26amp%3B%20Ruiz%20de%20Galarreta%2C%20J.%20I.%20%282015%29.%20Phytochemicals%20determination%20and%20classification%20in%20purple%20and%20red%20fleshed%20potato%20tubers%20by%20analytical%20methods%20and%20near%20infrared%20spectroscopy%3A%20Phytochemicals%20in%20potato%20tubers.%20%3Ci%3EJournal%20of%20the%20Science%20of%20Food%20and%20Agriculture%3C%5C%2Fi%3E%2C%20n%5C%2Fa-n%5C%2Fa.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fjsfa.7294%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fjsfa.7294%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Phytochemicals%20determination%20and%20classification%20in%20purple%20and%20red%20fleshed%20potato%20tubers%20by%20analytical%20methods%20and%20near%20infrared%20spectroscopy%3A%20Phytochemicals%20in%20potato%20tubers%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Roberto%22%2C%22lastName%22%3A%22Tierno%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ainara%22%2C%22lastName%22%3A%22L%5Cu00f3pez%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22Riga%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Silvia%22%2C%22lastName%22%3A%22Arazuri%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Carmen%22%2C%22lastName%22%3A%22Jar%5Cu00e9n%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Leire%22%2C%22lastName%22%3A%22Benedicto%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jos%5Cu00e9%20I%22%2C%22lastName%22%3A%22Ruiz%20de%20Galarreta%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2207%5C%2F2015%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1002%5C%2Fjsfa.7294%22%2C%22ISSN%22%3A%2200225142%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdoi.wiley.com%5C%2F10.1002%5C%2Fjsfa.7294%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-08-10T15%3A51%3A25Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Potato%22%7D%2C%7B%22tag%22%3A%22Small%20molecules%22%7D%2C%7B%22tag%22%3A%22Stems-roots-tubers%22%7D%2C%7B%22tag%22%3A%22Tubers%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22ES98BIII%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kraaijeveld%20et%20al.%22%2C%22parsedDate%22%3A%222015%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EKraaijeveld%2C%20K.%2C%20de%20Weger%2C%20L.%20A.%2C%20Ventayol%20Garc%26%23xED%3Ba%2C%20M.%2C%20Buermans%2C%20H.%2C%20Frank%2C%20J.%2C%20Hiemstra%2C%20P.%20S.%2C%20%26amp%3B%20den%20Dunnen%2C%20J.%20T.%20%282015%29.%20Efficient%20and%20sensitive%20identification%20and%20quantification%20of%20airborne%20pollen%20using%20next-generation%20DNA%20sequencing.%20%3Ci%3EMolecular%20Ecology%20Resources%3C%5C%2Fi%3E%2C%20%3Ci%3E15%3C%5C%2Fi%3E%281%29%2C%208%26%23x2013%3B16.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2F1755-0998.12288%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2F1755-0998.12288%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Efficient%20and%20sensitive%20identification%20and%20quantification%20of%20airborne%20pollen%20using%20next-generation%20DNA%20sequencing%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ken%22%2C%22lastName%22%3A%22Kraaijeveld%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Letty%20A.%22%2C%22lastName%22%3A%22de%20Weger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marina%22%2C%22lastName%22%3A%22Ventayol%20Garc%5Cu00eda%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Henk%22%2C%22lastName%22%3A%22Buermans%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jeroen%22%2C%22lastName%22%3A%22Frank%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pieter%20S.%22%2C%22lastName%22%3A%22Hiemstra%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Johan%20T.%22%2C%22lastName%22%3A%22den%20Dunnen%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2201%5C%2F2015%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1111%5C%2F1755-0998.12288%22%2C%22ISSN%22%3A%221755098X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdoi.wiley.com%5C%2F10.1111%5C%2F1755-0998.12288%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-31T17%3A29%3A35Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22DNA%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Pollen%22%7D%5D%7D%7D%2C%7B%22key%22%3A%225DGRTT9W%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mora%20et%20al.%22%2C%22parsedDate%22%3A%222014-09-15%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EMora%2C%20Y.%2C%20Diaz%2C%20R.%2C%20Vargas-Lagunas%2C%20C.%2C%20Peralta%2C%20H.%2C%20Guerrero%2C%20G.%2C%20Aguilar%2C%20A.%2C%20Encarnacion%2C%20S.%2C%20Girard%2C%20L.%2C%20%26amp%3B%20Mora%2C%20J.%20%282014%29.%20Nitrogen-Fixing%20Rhizobial%20Strains%20Isolated%20from%20Common%20Bean%20Seeds%3A%20Phylogeny%2C%20Physiology%2C%20and%20Genome%20Analysis.%20%3Ci%3EApplied%20and%20Environmental%20Microbiology%3C%5C%2Fi%3E%2C%20%3Ci%3E80%3C%5C%2Fi%3E%2818%29%2C%205644%26%23x2013%3B5654.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1128%5C%2FAEM.01491-14%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1128%5C%2FAEM.01491-14%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Nitrogen-Fixing%20Rhizobial%20Strains%20Isolated%20from%20Common%20Bean%20Seeds%3A%20Phylogeny%2C%20Physiology%2C%20and%20Genome%20Analysis%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Y.%22%2C%22lastName%22%3A%22Mora%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%22%2C%22lastName%22%3A%22Diaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%22%2C%22lastName%22%3A%22Vargas-Lagunas%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22H.%22%2C%22lastName%22%3A%22Peralta%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22G.%22%2C%22lastName%22%3A%22Guerrero%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Aguilar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%22%2C%22lastName%22%3A%22Encarnacion%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22L.%22%2C%22lastName%22%3A%22Girard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%22%2C%22lastName%22%3A%22Mora%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222014-09-15%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1128%5C%2FAEM.01491-14%22%2C%22ISSN%22%3A%220099-2240%2C%201098-5336%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Faem.asm.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1128%5C%2FAEM.01491-14%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-01-12T18%3A46%3A26Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Bacteria-viruses-organelles%22%7D%2C%7B%22tag%22%3A%22Bean%22%7D%2C%7B%22tag%22%3A%22Live%20bacteria%22%7D%2C%7B%22tag%22%3A%22Phaseolus%20vulgaris%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Seeds%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22NAKN3A7U%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Devanathan%20et%20al.%22%2C%22parsedDate%22%3A%222014-04-23%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EDevanathan%2C%20S.%2C%20Erban%2C%20A.%2C%20Perez-Torres%2C%20R.%2C%20Kopka%2C%20J.%2C%20%26amp%3B%20Makaroff%2C%20C.%20A.%20%282014%29.%20Arabidopsis%20thaliana%20Glyoxalase%202-1%20Is%20Required%20during%20Abiotic%20Stress%20but%20Is%20Not%20Essential%20under%20Normal%20Plant%20Growth.%20%3Ci%3EPLoS%20ONE%3C%5C%2Fi%3E%2C%20%3Ci%3E9%3C%5C%2Fi%3E%284%29%2C%20e95971.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0095971%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0095971%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Arabidopsis%20thaliana%20Glyoxalase%202-1%20Is%20Required%20during%20Abiotic%20Stress%20but%20Is%20Not%20Essential%20under%20Normal%20Plant%20Growth%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sriram%22%2C%22lastName%22%3A%22Devanathan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alexander%22%2C%22lastName%22%3A%22Erban%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rodolfo%22%2C%22lastName%22%3A%22Perez-Torres%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Joachim%22%2C%22lastName%22%3A%22Kopka%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christopher%20A.%22%2C%22lastName%22%3A%22Makaroff%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Haibing%22%2C%22lastName%22%3A%22Yang%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222014-4-23%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.pone.0095971%22%2C%22ISSN%22%3A%221932-6203%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.plos.org%5C%2F10.1371%5C%2Fjournal.pone.0095971%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-10-30T19%3A56%3A10Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Arabidopsis%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Seedlings%22%7D%2C%7B%22tag%22%3A%22Small%20molecules%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22G4JWBTEW%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Riga%20et%20al.%22%2C%22parsedDate%22%3A%222014%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ERiga%2C%20P.%2C%20Medina%2C%20S.%2C%20Garc%26%23xED%3Ba-Flores%2C%20L.%20A.%2C%20%26amp%3B%20Gil-Izquierdo%2C%20%26%23xC1%3B.%20%282014%29.%20Melatonin%20content%20of%20pepper%20and%20tomato%20fruits%3A%20Effects%20of%20cultivar%20and%20solar%20radiation.%20%3Ci%3EFood%20Chemistry%3C%5C%2Fi%3E%2C%20%3Ci%3E156%3C%5C%2Fi%3E%2C%20347%26%23x2013%3B352.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.foodchem.2014.01.117%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.foodchem.2014.01.117%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Melatonin%20content%20of%20pepper%20and%20tomato%20fruits%3A%20Effects%20of%20cultivar%20and%20solar%20radiation%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22Riga%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sonia%22%2C%22lastName%22%3A%22Medina%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Libia%20Alejandra%22%2C%22lastName%22%3A%22Garc%5Cu00eda-Flores%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22%5Cu00c1ngel%22%2C%22lastName%22%3A%22Gil-Izquierdo%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2208%5C%2F2014%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.foodchem.2014.01.117%22%2C%22ISSN%22%3A%2203088146%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS0308814614001599%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-08-05T13%3A15%3A13Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Fruit%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Pepper%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Small%20molecules%22%7D%2C%7B%22tag%22%3A%22Tomato%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22XZKU2M8T%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Shen%20et%20al.%22%2C%22parsedDate%22%3A%222014%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EShen%2C%20Y.-H.%2C%20Chen%2C%20Y.-H.%2C%20Liu%2C%20H.-Y.%2C%20Chiang%2C%20F.-Y.%2C%20Wang%2C%20Y.-C.%2C%20Hou%2C%20L.-Y.%2C%20Lin%2C%20J.-S.%2C%20Lin%2C%20C.-C.%2C%20Lin%2C%20H.-H.%2C%20Lai%2C%20H.-M.%2C%20%26amp%3B%20Jeng%2C%20S.-T.%20%282014%29.%20Expression%20of%20a%20gene%20encoding%20%26%23x3B2%3B-ureidopropionase%20is%20critical%20for%20pollen%20germination%20in%20tomatoes.%20%3Ci%3EPhysiologia%20Plantarum%3C%5C%2Fi%3E%2C%20%3Ci%3E150%3C%5C%2Fi%3E%283%29%2C%20425%26%23x2013%3B435.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2Fppl.12085%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2Fppl.12085%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Expression%20of%20a%20gene%20encoding%20%5Cu03b2-ureidopropionase%20is%20critical%20for%20pollen%20germination%20in%20tomatoes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yu-Hsing%22%2C%22lastName%22%3A%22Shen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yu-Huei%22%2C%22lastName%22%3A%22Chen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hao-Yun%22%2C%22lastName%22%3A%22Liu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fang-Yi%22%2C%22lastName%22%3A%22Chiang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yi-Chieh%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Liang-Yu%22%2C%22lastName%22%3A%22Hou%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jeng-Shane%22%2C%22lastName%22%3A%22Lin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chih-Ching%22%2C%22lastName%22%3A%22Lin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hsin-Hung%22%2C%22lastName%22%3A%22Lin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hsi-Mei%22%2C%22lastName%22%3A%22Lai%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shih-Tong%22%2C%22lastName%22%3A%22Jeng%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2203%5C%2F2014%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1111%5C%2Fppl.12085%22%2C%22ISSN%22%3A%2200319317%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdoi.wiley.com%5C%2F10.1111%5C%2Fppl.12085%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-10-30T20%3A39%3A52Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Flowers%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Small%20molecules%22%7D%2C%7B%22tag%22%3A%22Tomato%22%7D%5D%7D%7D%2C%7B%22key%22%3A%227VFMMCHM%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mohammad%22%2C%22parsedDate%22%3A%222013-04-01%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EMohammad.%20%282013%29.%20ELECTROPHORETIC%20ANALYSIS%20OF%20PROTEIN%20PATTERNS%20IN%20DATE%20PALM%20%26%23x201C%3BKHALAS%26%23x201D%3B%20CULTIVAR%20LEAFLETS%20AMONG%20DIFFERENT%20LOCATIONS%20OF%20AL-AHSA.%20%3Ci%3EAmerican%20Journal%20of%20Agricultural%20and%20Biological%20Sciences%3C%5C%2Fi%3E%2C%20%3Ci%3E8%3C%5C%2Fi%3E%284%29%2C%20343%26%23x2013%3B349.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3844%5C%2Fajabssp.2013.343.349%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3844%5C%2Fajabssp.2013.343.349%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22ELECTROPHORETIC%20ANALYSIS%20OF%20PROTEIN%20PATTERNS%20IN%20DATE%20PALM%20%5Cu201cKHALAS%5Cu201d%20CULTIVAR%20LEAFLETS%20AMONG%20DIFFERENT%20LOCATIONS%20OF%20AL-AHSA%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Mohammad%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222013-04-01%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.3844%5C%2Fajabssp.2013.343.349%22%2C%22ISSN%22%3A%221557-4989%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fthescipub.com%5C%2Fabstract%5C%2F10.3844%5C%2Fajabssp.2013.343.349%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-08-05T17%3A11%3A59Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Leaves%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Phoenix%20dactylifera%20L.%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22SDN9IJWX%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Wu%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EWu%2C%20P.%20H.%2C%20Liu%2C%20C.%20H.%2C%20Tseng%2C%20K.%20M.%2C%20Liu%2C%20Y.%20C.%2C%20Chen%2C%20C.%20C.%2C%20Yang%2C%20P.%20P.%2C%20Huang%2C%20Y.%20X.%2C%20Chen%2C%20W.%20H.%2C%20%26amp%3B%20Wang%2C%20H.%20L.%20%282013%29.%20Low%20irradiance%20alters%20carbon%20metabolism%20and%20delays%20flower%20stalk%20development%20in%20two%20orchids.%20%3Ci%3EBiologia%20Plantarum%3C%5C%2Fi%3E%2C%20%3Ci%3E57%3C%5C%2Fi%3E%284%29%2C%20764%26%23x2013%3B768.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs10535-013-0340-2%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs10535-013-0340-2%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Low%20irradiance%20alters%20carbon%20metabolism%20and%20delays%20flower%20stalk%20development%20in%20two%20orchids%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%20H.%22%2C%22lastName%22%3A%22Wu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%20H.%22%2C%22lastName%22%3A%22Liu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22K.%20M.%22%2C%22lastName%22%3A%22Tseng%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Y.%20C.%22%2C%22lastName%22%3A%22Liu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%20C.%22%2C%22lastName%22%3A%22Chen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%20P.%22%2C%22lastName%22%3A%22Yang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Y.%20X.%22%2C%22lastName%22%3A%22Huang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22W.%20H.%22%2C%22lastName%22%3A%22Chen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22H.%20L.%22%2C%22lastName%22%3A%22Wang%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2212%5C%2F2013%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1007%5C%2Fs10535-013-0340-2%22%2C%22ISSN%22%3A%220006-3134%2C%201573-8264%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flink.springer.com%5C%2F10.1007%5C%2Fs10535-013-0340-2%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T15%3A39%3A26Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Leaves%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Orchid%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%5D%7D%7D%2C%7B%22key%22%3A%226PQFWTW8%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kwon%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EKwon%2C%20D.%20H.%2C%20Park%2C%20J.%20H.%2C%20%26amp%3B%20Lee%2C%20S.%20H.%20%282013%29.%20Screening%20of%20lethal%20genes%20for%20feeding%20RNAi%20by%20leaf%20disc-mediated%20systematic%20delivery%20of%20dsRNA%20in%20Tetranychus%20urticae.%20%3Ci%3EPesticide%20Biochemistry%20and%20Physiology%3C%5C%2Fi%3E%2C%20%3Ci%3E105%3C%5C%2Fi%3E%281%29%2C%2069%26%23x2013%3B75.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.pestbp.2012.12.001%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.pestbp.2012.12.001%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Screening%20of%20lethal%20genes%20for%20feeding%20RNAi%20by%20leaf%20disc-mediated%20systematic%20delivery%20of%20dsRNA%20in%20Tetranychus%20urticae%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Deok%20Ho%22%2C%22lastName%22%3A%22Kwon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ji%20Hyun%22%2C%22lastName%22%3A%22Park%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Si%20Hyeock%22%2C%22lastName%22%3A%22Lee%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%221%5C%2F2013%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.pestbp.2012.12.001%22%2C%22ISSN%22%3A%2200483575%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS0048357512001824%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-08T16%3A29%3A15Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Insects%22%7D%2C%7B%22tag%22%3A%22Insects-S%22%7D%2C%7B%22tag%22%3A%22Leaves%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Phaseolus%20vulgaris%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22RNA%22%7D%2C%7B%22tag%22%3A%22Small%20organisms%22%7D%2C%7B%22tag%22%3A%22Tetranychus%20urticae%22%7D%5D%7D%7D%2C%7B%22key%22%3A%223Z6I4KBV%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Qualley%20et%20al.%22%2C%22parsedDate%22%3A%222012-10-02%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EQualley%2C%20A.%20V.%2C%20Widhalm%2C%20J.%20R.%2C%20Adebesin%2C%20F.%2C%20Kish%2C%20C.%20M.%2C%20%26amp%3B%20Dudareva%2C%20N.%20%282012%29.%20Completion%20of%20the%20core%20-oxidative%20pathway%20of%20benzoic%20acid%20biosynthesis%20in%20plants.%20%3Ci%3EProceedings%20of%20the%20National%20Academy%20of%20Sciences%3C%5C%2Fi%3E%2C%20%3Ci%3E109%3C%5C%2Fi%3E%2840%29%2C%2016383%26%23x2013%3B16388.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1073%5C%2Fpnas.1211001109%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1073%5C%2Fpnas.1211001109%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Completion%20of%20the%20core%20-oxidative%20pathway%20of%20benzoic%20acid%20biosynthesis%20in%20plants%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%20V.%22%2C%22lastName%22%3A%22Qualley%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20R.%22%2C%22lastName%22%3A%22Widhalm%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22F.%22%2C%22lastName%22%3A%22Adebesin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%20M.%22%2C%22lastName%22%3A%22Kish%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22N.%22%2C%22lastName%22%3A%22Dudareva%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222012-10-02%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1073%5C%2Fpnas.1211001109%22%2C%22ISSN%22%3A%220027-8424%2C%201091-6490%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.pnas.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1073%5C%2Fpnas.1211001109%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T15%3A47%3A44Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Flowers%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Petunia%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Small%20molecules%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22JB2QRA5A%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mamedov%20et%20al.%22%2C%22parsedDate%22%3A%222012%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EMamedov%2C%20T.%2C%20Ghosh%2C%20A.%2C%20Jones%2C%20R.%20M.%2C%20Mett%2C%20V.%2C%20Farrance%2C%20C.%20E.%2C%20Musiychuk%2C%20K.%2C%20Horsey%2C%20A.%2C%20%26amp%3B%20Yusibov%2C%20V.%20%282012%29.%20Production%20of%20non-glycosylated%20recombinant%20proteins%20in%20%3Ci%3ENicotiana%20benthamiana%3C%5C%2Fi%3E%20plants%20by%20co-expressing%20bacterial%20PNGase%20F%3A%20Production%20of%20non-glycosylated%20recombinant%20proteins%20in%20%3Ci%3ENicotiana%20benthamiana%3C%5C%2Fi%3E.%20%3Ci%3EPlant%20Biotechnology%20Journal%3C%5C%2Fi%3E%2C%20%3Ci%3E10%3C%5C%2Fi%3E%287%29%2C%20773%26%23x2013%3B782.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2Fj.1467-7652.2012.00694.x%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2Fj.1467-7652.2012.00694.x%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Production%20of%20non-glycosylated%20recombinant%20proteins%20in%20%3Ci%3ENicotiana%20benthamiana%3C%5C%2Fi%3E%20plants%20by%20co-expressing%20bacterial%20PNGase%20F%3A%20Production%20of%20non-glycosylated%20recombinant%20proteins%20in%20%3Ci%3ENicotiana%20benthamiana%3C%5C%2Fi%3E%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tarlan%22%2C%22lastName%22%3A%22Mamedov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ananya%22%2C%22lastName%22%3A%22Ghosh%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20Mark%22%2C%22lastName%22%3A%22Jones%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Vadim%22%2C%22lastName%22%3A%22Mett%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christine%20E.%22%2C%22lastName%22%3A%22Farrance%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Konstantin%22%2C%22lastName%22%3A%22Musiychuk%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22April%22%2C%22lastName%22%3A%22Horsey%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Vidadi%22%2C%22lastName%22%3A%22Yusibov%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2209%5C%2F2012%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1111%5C%2Fj.1467-7652.2012.00694.x%22%2C%22ISSN%22%3A%2214677644%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdoi.wiley.com%5C%2F10.1111%5C%2Fj.1467-7652.2012.00694.x%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T15%3A24%3A31Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Leaves%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%2C%7B%22tag%22%3A%22Tobacco%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22B7SG2HRZ%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Augustine%20et%20al.%22%2C%22parsedDate%22%3A%222011-10-01%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EAugustine%2C%20R.%20C.%2C%20Pattavina%2C%20K.%20A.%2C%20Tuzel%2C%20E.%2C%20Vidali%2C%20L.%2C%20%26amp%3B%20Bezanilla%2C%20M.%20%282011%29.%20Actin%20Interacting%20Protein1%20and%20Actin%20Depolymerizing%20Factor%20Drive%20Rapid%20Actin%20Dynamics%20in%20Physcomitrella%20patens.%20%3Ci%3EThe%20Plant%20Cell%3C%5C%2Fi%3E%2C%20%3Ci%3E23%3C%5C%2Fi%3E%2810%29%2C%203696%26%23x2013%3B3710.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1105%5C%2Ftpc.111.090753%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1105%5C%2Ftpc.111.090753%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Actin%20Interacting%20Protein1%20and%20Actin%20Depolymerizing%20Factor%20Drive%20Rapid%20Actin%20Dynamics%20in%20Physcomitrella%20patens%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20C.%22%2C%22lastName%22%3A%22Augustine%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22K.%20A.%22%2C%22lastName%22%3A%22Pattavina%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22E.%22%2C%22lastName%22%3A%22Tuzel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22L.%22%2C%22lastName%22%3A%22Vidali%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Bezanilla%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222011-10-01%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1105%5C%2Ftpc.111.090753%22%2C%22ISSN%22%3A%221040-4651%2C%201532-298X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.plantcell.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1105%5C%2Ftpc.111.090753%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T15%3A46%3A14Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22DNA%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Moss%22%7D%2C%7B%22tag%22%3A%22Physcomitrella%20patens%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22FWXXH9UG%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mamedov%20and%20Yusibov%22%2C%22parsedDate%22%3A%222011%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EMamedov%2C%20T.%2C%20%26amp%3B%20Yusibov%2C%20V.%20%282011%29.%20Green%20algae%20Chlamydomonas%20reinhardtii%20possess%20endogenous%20sialylated%20N-glycans.%20%3Ci%3EFEBS%20Open%20Bio%3C%5C%2Fi%3E%2C%20%3Ci%3E1%3C%5C%2Fi%3E%2C%2015%26%23x2013%3B22.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.fob.2011.10.003%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.fob.2011.10.003%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Green%20algae%20Chlamydomonas%20reinhardtii%20possess%20endogenous%20sialylated%20N-glycans%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tarlan%22%2C%22lastName%22%3A%22Mamedov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Vidadi%22%2C%22lastName%22%3A%22Yusibov%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2212%5C%2F2011%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.fob.2011.10.003%22%2C%22ISSN%22%3A%2222115463%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS2211546311000040%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T15%3A31%3A18Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Chlamydomonas%20reinhardtii%22%7D%2C%7B%22tag%22%3A%22Leaves%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%2C%7B%22tag%22%3A%22Tobacco%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22KSRTIKWB%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rommens%20et%20al.%22%2C%22parsedDate%22%3A%222010-03-24%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ERommens%2C%20C.%20M.%2C%20Shakya%2C%20R.%2C%20Heap%2C%20M.%2C%20%26amp%3B%20Fessenden%2C%20K.%20%282010%29.%20Tastier%20and%20Healthier%20Alternatives%20to%20French%20Fries.%20%3Ci%3EJournal%20of%20Food%20Science%3C%5C%2Fi%3E%2C%20%3Ci%3E75%3C%5C%2Fi%3E%284%29%2C%20H109%26%23x2013%3BH115.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2Fj.1750-3841.2010.01588.x%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2Fj.1750-3841.2010.01588.x%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Tastier%20and%20Healthier%20Alternatives%20to%20French%20Fries%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Caius%20M.%22%2C%22lastName%22%3A%22Rommens%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Roshani%22%2C%22lastName%22%3A%22Shakya%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mark%22%2C%22lastName%22%3A%22Heap%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kristi%22%2C%22lastName%22%3A%22Fessenden%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222010-03-24%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1111%5C%2Fj.1750-3841.2010.01588.x%22%2C%22ISSN%22%3A%2200221147%2C%2017503841%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdoi.wiley.com%5C%2F10.1111%5C%2Fj.1750-3841.2010.01588.x%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T15%3A56%3A15Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Lipids%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Potato%22%7D%2C%7B%22tag%22%3A%22Stems-roots-tubers%22%7D%5D%7D%7D%5D%7D
Nguyen, H. M., Yadav, N. S., Barak, S., Lima, F. P., Sapir, Y., & Winters, G. (2020). Responses of Invasive and Native Populations of the Seagrass Halophila stipulacea to Simulated Climate Change. Frontiers in Marine Science, 6, 812. https://doi.org/10.3389/fmars.2019.00812
Diamos, A. G., Rosenthal, S. H., & Mason, H. S. (2016). 5′ and 3′ Untranslated Regions Strongly Enhance Performance of Geminiviral Replicons in Nicotiana benthamiana Leaves. Frontiers in Plant Science, 7. https://doi.org/10.3389/fpls.2016.00200
Riga, P., Benedicto, L., García-Flores, L., Villaño, D., Medina, S., & Gil-Izquierdo, Á. (2016). Rootstock effect on serotonin and nutritional quality of tomatoes produced under low temperature and light conditions. Journal of Food Composition and Analysis, 46, 50–59. https://doi.org/10.1016/j.jfca.2015.11.003
Davis, T. S., Bosque-Pérez, N. A., Popova, I., & Eigenbrode, S. D. (2015). Evidence for additive effects of virus infection and water availability on phytohormone induction in a staple crop. Frontiers in Ecology and Evolution, 3. https://doi.org/10.3389/fevo.2015.00114
Mohammad, A.-I. A. (2015). Electrophoretic analysis of proteins from different date palm (Phoenix dactylifera L.) cultivars in Saudi Arabia. African Journal of Biotechnology, 14(15), 1325–1333. https://doi.org/10.5897/AJB2014.14398
Tierno, R., López, A., Riga, P., Arazuri, S., Jarén, C., Benedicto, L., & Ruiz de Galarreta, J. I. (2015). Phytochemicals determination and classification in purple and red fleshed potato tubers by analytical methods and near infrared spectroscopy: Phytochemicals in potato tubers. Journal of the Science of Food and Agriculture, n/a-n/a. https://doi.org/10.1002/jsfa.7294
Kraaijeveld, K., de Weger, L. A., Ventayol García, M., Buermans, H., Frank, J., Hiemstra, P. S., & den Dunnen, J. T. (2015). Efficient and sensitive identification and quantification of airborne pollen using next-generation DNA sequencing. Molecular Ecology Resources, 15(1), 8–16. https://doi.org/10.1111/1755-0998.12288
Mora, Y., Diaz, R., Vargas-Lagunas, C., Peralta, H., Guerrero, G., Aguilar, A., Encarnacion, S., Girard, L., & Mora, J. (2014). Nitrogen-Fixing Rhizobial Strains Isolated from Common Bean Seeds: Phylogeny, Physiology, and Genome Analysis. Applied and Environmental Microbiology, 80(18), 5644–5654. https://doi.org/10.1128/AEM.01491-14
Devanathan, S., Erban, A., Perez-Torres, R., Kopka, J., & Makaroff, C. A. (2014). Arabidopsis thaliana Glyoxalase 2-1 Is Required during Abiotic Stress but Is Not Essential under Normal Plant Growth. PLoS ONE, 9(4), e95971. https://doi.org/10.1371/journal.pone.0095971
Riga, P., Medina, S., García-Flores, L. A., & Gil-Izquierdo, Á. (2014). Melatonin content of pepper and tomato fruits: Effects of cultivar and solar radiation. Food Chemistry, 156, 347–352. https://doi.org/10.1016/j.foodchem.2014.01.117
Shen, Y.-H., Chen, Y.-H., Liu, H.-Y., Chiang, F.-Y., Wang, Y.-C., Hou, L.-Y., Lin, J.-S., Lin, C.-C., Lin, H.-H., Lai, H.-M., & Jeng, S.-T. (2014). Expression of a gene encoding β-ureidopropionase is critical for pollen germination in tomatoes. Physiologia Plantarum, 150(3), 425–435. https://doi.org/10.1111/ppl.12085
Mohammad. (2013). ELECTROPHORETIC ANALYSIS OF PROTEIN PATTERNS IN DATE PALM “KHALAS” CULTIVAR LEAFLETS AMONG DIFFERENT LOCATIONS OF AL-AHSA. American Journal of Agricultural and Biological Sciences, 8(4), 343–349. https://doi.org/10.3844/ajabssp.2013.343.349
Wu, P. H., Liu, C. H., Tseng, K. M., Liu, Y. C., Chen, C. C., Yang, P. P., Huang, Y. X., Chen, W. H., & Wang, H. L. (2013). Low irradiance alters carbon metabolism and delays flower stalk development in two orchids. Biologia Plantarum, 57(4), 764–768. https://doi.org/10.1007/s10535-013-0340-2
Kwon, D. H., Park, J. H., & Lee, S. H. (2013). Screening of lethal genes for feeding RNAi by leaf disc-mediated systematic delivery of dsRNA in Tetranychus urticae. Pesticide Biochemistry and Physiology, 105(1), 69–75. https://doi.org/10.1016/j.pestbp.2012.12.001
Qualley, A. V., Widhalm, J. R., Adebesin, F., Kish, C. M., & Dudareva, N. (2012). Completion of the core -oxidative pathway of benzoic acid biosynthesis in plants. Proceedings of the National Academy of Sciences, 109(40), 16383–16388. https://doi.org/10.1073/pnas.1211001109
Mamedov, T., Ghosh, A., Jones, R. M., Mett, V., Farrance, C. E., Musiychuk, K., Horsey, A., & Yusibov, V. (2012). Production of non-glycosylated recombinant proteins in Nicotiana benthamiana plants by co-expressing bacterial PNGase F: Production of non-glycosylated recombinant proteins in Nicotiana benthamiana. Plant Biotechnology Journal, 10(7), 773–782. https://doi.org/10.1111/j.1467-7652.2012.00694.x
Augustine, R. C., Pattavina, K. A., Tuzel, E., Vidali, L., & Bezanilla, M. (2011). Actin Interacting Protein1 and Actin Depolymerizing Factor Drive Rapid Actin Dynamics in Physcomitrella patens. The Plant Cell, 23(10), 3696–3710. https://doi.org/10.1105/tpc.111.090753
Mamedov, T., & Yusibov, V. (2011). Green algae Chlamydomonas reinhardtii possess endogenous sialylated N-glycans. FEBS Open Bio, 1, 15–22. https://doi.org/10.1016/j.fob.2011.10.003
Rommens, C. M., Shakya, R., Heap, M., & Fessenden, K. (2010). Tastier and Healthier Alternatives to French Fries. Journal of Food Science, 75(4), H109–H115. https://doi.org/10.1111/j.1750-3841.2010.01588.x
Algae
2474232
algae
1
apa
50
date
desc
1
3227
https://www.nextadvance.com/wp-content/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22BZ79VMPU%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Thapa%20et%20al.%22%2C%22parsedDate%22%3A%222016-04-06%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EThapa%2C%20H.%20R.%2C%20Naik%2C%20M.%20T.%2C%20Okada%2C%20S.%2C%20Takada%2C%20K.%2C%20Moln%26%23xE1%3Br%2C%20I.%2C%20Xu%2C%20Y.%2C%20%26amp%3B%20Devarenne%2C%20T.%20P.%20%282016%29.%20A%20squalene%20synthase-like%20enzyme%20initiates%20production%20of%20tetraterpenoid%20hydrocarbons%20in%20Botryococcus%20braunii%20Race%20L.%20%3Ci%3ENature%20Communications%3C%5C%2Fi%3E%2C%20%3Ci%3E7%3C%5C%2Fi%3E%2C%2011198.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fncomms11198%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fncomms11198%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20squalene%20synthase-like%20enzyme%20initiates%20production%20of%20tetraterpenoid%20hydrocarbons%20in%20Botryococcus%20braunii%20Race%20L%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hem%20R.%22%2C%22lastName%22%3A%22Thapa%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mandar%20T.%22%2C%22lastName%22%3A%22Naik%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shigeru%22%2C%22lastName%22%3A%22Okada%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kentaro%22%2C%22lastName%22%3A%22Takada%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Istv%5Cu00e1n%22%2C%22lastName%22%3A%22Moln%5Cu00e1r%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yuquan%22%2C%22lastName%22%3A%22Xu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Timothy%20P.%22%2C%22lastName%22%3A%22Devarenne%22%7D%5D%2C%22abstractNote%22%3A%22The%20green%20microalga%20Botryococcus%20braunii%20is%20considered%20a%20promising%20biofuel%20feedstock%20producer%20due%20to%20its%20prodigious%20accumulation%20of%20hydrocarbon%20oils%20that%20can%20be%20converted%20into%20fuels.%20B.%20braunii%20Race%20L%20produces%20the%20C40%20tetraterpenoid%20hydrocarbon%20lycopadiene%20via%20an%20uncharacterized%20biosynthetic%20pathway.%20Structural%20similarities%20suggest%20this%20pathway%20follows%20a%20biosynthetic%20mechanism%20analogous%20to%20that%20of%20C30%20squalene.%20Confirming%20this%20hypothesis%2C%20the%20current%20study%20identifies%20C20%20geranylgeranyl%20diphosphate%20%28GGPP%29%20as%20a%20precursor%20for%20lycopaoctaene%20biosynthesis%2C%20the%20first%20committed%20intermediate%20in%20the%20production%20of%20lycopadiene.%20Two%20squalene%20synthase%20%28SS%29-like%20complementary%20DNAs%20are%20identified%20in%20race%20L%20with%20one%20encoding%20a%20true%20SS%20and%20the%20other%20encoding%20an%20enzyme%20with%20lycopaoctaene%20synthase%20%28LOS%29%20activity.%20Interestingly%2C%20LOS%20uses%20alternative%20C15%20and%20C20%20prenyl%20diphosphate%20substrates%20to%20produce%20combinatorial%20hybrid%20hydrocarbons%2C%20but%20almost%20exclusively%20uses%20GGPP%20in%20vivo.%20This%20discovery%20highlights%20how%20SS%20enzyme%20diversification%20results%20in%20the%20production%20of%20specialized%20tetraterpenoid%20oils%20in%20race%20L%20of%20B.%20braunii.%22%2C%22date%22%3A%22April%206%2C%202016%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1038%5C%2Fncomms11198%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.nature.com%5C%2Fncomms%5C%2F2016%5C%2F160406%5C%2Fncomms11198%5C%2Ffull%5C%2Fncomms11198.html%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-06-24T16%3A43%3A13Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Algae%22%7D%2C%7B%22tag%22%3A%22Botryococcus%22%7D%2C%7B%22tag%22%3A%22Cultured%20cells%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22HSETCS8Q%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kent%20et%20al.%22%2C%22parsedDate%22%3A%222015-02-27%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EKent%2C%20M.%2C%20Welladsen%2C%20H.%20M.%2C%20Mangott%2C%20A.%2C%20%26amp%3B%20Li%2C%20Y.%20%282015%29.%20Nutritional%20Evaluation%20of%20Australian%20Microalgae%20as%20Potential%20Human%20Health%20Supplements.%20%3Ci%3EPLOS%20ONE%3C%5C%2Fi%3E%2C%20%3Ci%3E10%3C%5C%2Fi%3E%282%29%2C%20e0118985.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0118985%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0118985%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Nutritional%20Evaluation%20of%20Australian%20Microalgae%20as%20Potential%20Human%20Health%20Supplements%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Megan%22%2C%22lastName%22%3A%22Kent%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Heather%20M.%22%2C%22lastName%22%3A%22Welladsen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Arnold%22%2C%22lastName%22%3A%22Mangott%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yan%22%2C%22lastName%22%3A%22Li%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Shashi%22%2C%22lastName%22%3A%22Kumar%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222015-2-27%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.pone.0118985%22%2C%22ISSN%22%3A%221932-6203%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.plos.org%5C%2F10.1371%5C%2Fjournal.pone.0118985%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-10-30T20%3A40%3A29Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Algae%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Protists%22%7D%2C%7B%22tag%22%3A%22Small%20molecules%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22UHI5U4P2%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Li%20et%20al.%22%2C%22parsedDate%22%3A%222015%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ELi%2C%20Y.%2C%20Xiao%2C%20G.%2C%20Mangott%2C%20A.%2C%20Kent%2C%20M.%2C%20%26amp%3B%20Pirozzi%2C%20I.%20%282015%29.%20Nutrient%20efficacy%20of%20microalgae%20as%20aquafeed%20additives%20for%20the%20adult%20black%20tiger%20prawn%2C%20%3Ci%3EPenaeus%20monodon%3C%5C%2Fi%3E.%20%3Ci%3EAquaculture%20Research%3C%5C%2Fi%3E%2C%20%3Ci%3E46%3C%5C%2Fi%3E.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2Fare.12815%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2Fare.12815%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Nutrient%20efficacy%20of%20microalgae%20as%20aquafeed%20additives%20for%20the%20adult%20black%20tiger%20prawn%2C%20%3Ci%3EPenaeus%20monodon%3C%5C%2Fi%3E%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yan%22%2C%22lastName%22%3A%22Li%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Guoqiang%22%2C%22lastName%22%3A%22Xiao%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Arnold%22%2C%22lastName%22%3A%22Mangott%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Megan%22%2C%22lastName%22%3A%22Kent%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Igor%22%2C%22lastName%22%3A%22Pirozzi%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2206%5C%2F2015%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1111%5C%2Fare.12815%22%2C%22ISSN%22%3A%221355557X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdoi.wiley.com%5C%2F10.1111%5C%2Fare.12815%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-12-30T14%3A38%3A31Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Algae%22%7D%2C%7B%22tag%22%3A%22Chlorella%22%7D%2C%7B%22tag%22%3A%22Dunaliella%22%7D%2C%7B%22tag%22%3A%22Entomoneis%22%7D%2C%7B%22tag%22%3A%22Melosira%22%7D%2C%7B%22tag%22%3A%22Microalgae%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Nannochloropsis%22%7D%2C%7B%22tag%22%3A%22Pigments%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Small%20molecules%22%7D%2C%7B%22tag%22%3A%22Stauroneis%22%7D%5D%7D%7D%2C%7B%22key%22%3A%2227HXMIS6%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22von%20Alvensleben%20et%20al.%22%2C%22parsedDate%22%3A%222013-05-07%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3Evon%20Alvensleben%2C%20N.%2C%20Stookey%2C%20K.%2C%20Magnusson%2C%20M.%2C%20%26amp%3B%20Heimann%2C%20K.%20%282013%29.%20Salinity%20Tolerance%20of%20Picochlorum%20atomus%20and%20the%20Use%20of%20Salinity%20for%20Contamination%20Control%20by%20the%20Freshwater%20Cyanobacterium%20Pseudanabaena%20limnetica.%20%3Ci%3EPLoS%20ONE%3C%5C%2Fi%3E%2C%20%3Ci%3E8%3C%5C%2Fi%3E%285%29%2C%20e63569.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0063569%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0063569%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Salinity%20Tolerance%20of%20Picochlorum%20atomus%20and%20the%20Use%20of%20Salinity%20for%20Contamination%20Control%20by%20the%20Freshwater%20Cyanobacterium%20Pseudanabaena%20limnetica%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22von%20Alvensleben%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Katherine%22%2C%22lastName%22%3A%22Stookey%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marie%22%2C%22lastName%22%3A%22Magnusson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kirsten%22%2C%22lastName%22%3A%22Heimann%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Lucas%20J.%22%2C%22lastName%22%3A%22Stal%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222013-5-7%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.pone.0063569%22%2C%22ISSN%22%3A%221932-6203%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.plos.org%5C%2F10.1371%5C%2Fjournal.pone.0063569%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T14%3A47%3A53Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Algae%22%7D%2C%7B%22tag%22%3A%22Carbohydrates%22%7D%2C%7B%22tag%22%3A%22Cell%20Suspension%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Picochlorum%20atomus%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22I67ECMAX%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Edwards%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EEdwards%2C%20C.%20D.%2C%20Beatty%2C%20J.%20C.%2C%20Loiselle%2C%20J.%20B.%20R.%2C%20Vlassov%2C%20K.%20A.%2C%20%26amp%3B%20Lefebvre%2C%20D.%20D.%20%282013%29.%20Aerobic%20transformation%20of%20zinc%20into%20metal%20sulfide%20by%20photosynthetic%20microorganisms.%20%3Ci%3EApplied%20Microbiology%20and%20Biotechnology%3C%5C%2Fi%3E%2C%20%3Ci%3E97%3C%5C%2Fi%3E%288%29%2C%203613%26%23x2013%3B3623.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs00253-012-4636-5%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs00253-012-4636-5%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Aerobic%20transformation%20of%20zinc%20into%20metal%20sulfide%20by%20photosynthetic%20microorganisms%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chad%20D.%22%2C%22lastName%22%3A%22Edwards%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Joseph%20C.%22%2C%22lastName%22%3A%22Beatty%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jacqueline%20B.%20R.%22%2C%22lastName%22%3A%22Loiselle%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Katya%20A.%22%2C%22lastName%22%3A%22Vlassov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daniel%20D.%22%2C%22lastName%22%3A%22Lefebvre%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%224%5C%2F2013%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1007%5C%2Fs00253-012-4636-5%22%2C%22ISSN%22%3A%220175-7598%2C%201432-0614%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flink.springer.com%5C%2F10.1007%5C%2Fs00253-012-4636-5%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T14%3A41%3A32Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Algae%22%7D%2C%7B%22tag%22%3A%22Cyanidioschyzon%20merolae%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22IE5AR8HT%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Edwards%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EEdwards%2C%20C.%20D.%2C%20Beatty%2C%20J.%20C.%2C%20Loiselle%2C%20J.%20B.%2C%20Vlassov%2C%20K.%20A.%2C%20%26amp%3B%20Lefebvre%2C%20D.%20D.%20%282013%29.%20Aerobic%20transformation%20of%20cadmium%20through%20metal%20sulfide%20biosynthesis%20in%20photosynthetic%20microorganisms.%20%3Ci%3EBMC%20Microbiology%3C%5C%2Fi%3E%2C%20%3Ci%3E13%3C%5C%2Fi%3E%281%29%2C%20161.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1186%5C%2F1471-2180-13-161%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1186%5C%2F1471-2180-13-161%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Aerobic%20transformation%20of%20cadmium%20through%20metal%20sulfide%20biosynthesis%20in%20photosynthetic%20microorganisms%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chad%20D%22%2C%22lastName%22%3A%22Edwards%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Joseph%20C%22%2C%22lastName%22%3A%22Beatty%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jacqueline%20BR%22%2C%22lastName%22%3A%22Loiselle%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Katya%20A%22%2C%22lastName%22%3A%22Vlassov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daniel%20D%22%2C%22lastName%22%3A%22Lefebvre%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222013%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1186%5C%2F1471-2180-13-161%22%2C%22ISSN%22%3A%221471-2180%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.biomedcentral.com%5C%2F1471-2180%5C%2F13%5C%2F161%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T14%3A36%3A46Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Algae%22%7D%2C%7B%22tag%22%3A%22Chlamydomonas%20reinhardtii%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%5D%7D%7D%5D%7D
Thapa, H. R., Naik, M. T., Okada, S., Takada, K., Molnár, I., Xu, Y., & Devarenne, T. P. (2016). A squalene synthase-like enzyme initiates production of tetraterpenoid hydrocarbons in Botryococcus braunii Race L. Nature Communications, 7, 11198. https://doi.org/10.1038/ncomms11198
Kent, M., Welladsen, H. M., Mangott, A., & Li, Y. (2015). Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLOS ONE, 10(2), e0118985. https://doi.org/10.1371/journal.pone.0118985
Li, Y., Xiao, G., Mangott, A., Kent, M., & Pirozzi, I. (2015). Nutrient efficacy of microalgae as aquafeed additives for the adult black tiger prawn, Penaeus monodon. Aquaculture Research, 46. https://doi.org/10.1111/are.12815
von Alvensleben, N., Stookey, K., Magnusson, M., & Heimann, K. (2013). Salinity Tolerance of Picochlorum atomus and the Use of Salinity for Contamination Control by the Freshwater Cyanobacterium Pseudanabaena limnetica. PLoS ONE, 8(5), e63569. https://doi.org/10.1371/journal.pone.0063569
Edwards, C. D., Beatty, J. C., Loiselle, J. B. R., Vlassov, K. A., & Lefebvre, D. D. (2013). Aerobic transformation of zinc into metal sulfide by photosynthetic microorganisms. Applied Microbiology and Biotechnology, 97(8), 3613–3623. https://doi.org/10.1007/s00253-012-4636-5
Edwards, C. D., Beatty, J. C., Loiselle, J. B., Vlassov, K. A., & Lefebvre, D. D. (2013). Aerobic transformation of cadmium through metal sulfide biosynthesis in photosynthetic microorganisms. BMC Microbiology, 13(1), 161. https://doi.org/10.1186/1471-2180-13-161
Fungi
2474232
fungi
1
apa
50
date
desc
1
3227
https://www.nextadvance.com/wp-content/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22GQ63XEV2%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rasool%20et%20al.%22%2C%22parsedDate%22%3A%222016-10-02%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ERasool%2C%20A.%2C%20Ahmed%2C%20M.%20S.%2C%20%26amp%3B%20Li%2C%20C.%20%282016%29.%20Overproduction%20of%20squalene%20synergistically%20downregulates%20ethanol%20production%20in%20Saccharomyces%20cerevisiae.%20%3Ci%3EChemical%20Engineering%20Science%3C%5C%2Fi%3E%2C%20%3Ci%3E152%3C%5C%2Fi%3E%2C%20370%26%23x2013%3B380.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ces.2016.06.014%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ces.2016.06.014%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Overproduction%20of%20squalene%20synergistically%20downregulates%20ethanol%20production%20in%20Saccharomyces%20cerevisiae%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aamir%22%2C%22lastName%22%3A%22Rasool%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Muhammad%20Saad%22%2C%22lastName%22%3A%22Ahmed%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chun%22%2C%22lastName%22%3A%22Li%22%7D%5D%2C%22abstractNote%22%3A%22Metabolic%20engineering%20strategies%20are%20often%20devised%20to%20redirect%20precursor%20flux%20in%20an%20engineered%20pathway.%20To%20develop%20a%20new%20strategy%2C%20the%20effect%20of%20overexpression%20of%20the%20squalene%20biosynthesis%20%28SB%29%20pathway%20and%20squalene%20overproduction%20on%20the%20ethanol%20production%20%28EP%29%20and%20post-squalene%20biosynthesis%20%28PB%29%20pathways%20was%20determined%20in%20Saccharomyces%20cerevisiae.%20Through%20overexpression%20of%20the%20HMG1%2C%20IDI1%2C%20ERG20%20and%20ERG9%20genes%20of%20the%20SB%20pathway%2C%20production%20of%20squalene%20increased%2010-fold%20in%20the%20M1EG%20strain%20compared%20to%20the%20wild-type%20strain%20%28WT%29%20%5B%2834%20mg%5C%2FL%29%2C%20without%20terbinafine%2C%20an%20inhibitor%20of%20squalene%20monooxygenase%2C%20and%2035.02-fold%20%28119.08%20mg%5C%2FL%29%20with%20terbinafine%5D.%20However%2C%20due%20to%20overexpression%20of%20the%20SB%20pathway%20and%20squalene%20overproduction%2C%20production%20of%20ethanol%20and%20functionality%20of%20the%20EP%20and%20PB%20pathways%20were%20synergistically%20downregulated%20by%2051.61%25%2C%2095.86%25%20and%2081.79%25%2C%20respectively%2C%20in%20the%20M1EG%20strain%20compared%20to%20the%20WT%20strain.%20Overexpression%20of%20the%20entire%20SB%20pathway%20also%20enhanced%20production%20of%20squalene%20by%2076.12-fold%20%28304.49%20mg%5C%2FL%29%20and%20synergistically%20downregulated%20production%20of%20ethanol%20and%20functionality%20of%20the%20EP%20and%20PB%20pathways%20by%2066.13%25%2C%2097.02%25%20and%2087.56%25%20in%20the%20FOH-2%20strain%20compared%20to%20the%20WT%20strain%2C%20respectively.%20The%20EP%20and%20PB%20pathways%20were%20strongly%20downregulated%20in%20the%20FOH-2%20strain%20compared%20to%20the%20M1EG%20strain%20because%20the%20FOH-2%20strain%20overexpresses%20the%20entire%20SB%20pathway%20and%20produces%20more%20squalene%20than%20the%20M1EG%20strain.%5Cn%5CnThese%20data%20suggest%20that%20overexpression%20of%20the%20SB%20pathway%20and%20squalene%20overproduction%20downregulate%20the%20EP%20and%20PB%20pathways%20in%20engineered%20strains.%20Therefore%2C%20we%20speculate%20that%20a%20cryptic%20regulation%20mechanism%20may%20downregulate%20these%20pathways%2C%20and%20characterization%20of%20such%20a%20mechanism%20may%20enable%20us%20to%20divert%20the%20precursor%20flux%20from%20the%20EP%20and%20PB%20pathways%20to%20the%20squalene%20biosynthesis%20pathway.%22%2C%22date%22%3A%22October%202%2C%202016%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.ces.2016.06.014%22%2C%22ISSN%22%3A%220009-2509%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fpii%5C%2FS0009250916303062%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-06-24T16%3A36%3A38Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Cultured%20cells%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-yeast%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Saccharomyces%20cerevisiae%22%7D%2C%7B%22tag%22%3A%22Small%20molecules%22%7D%2C%7B%22tag%22%3A%22Yeast%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22J26QJTXI%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Orgil%20et%20al.%22%2C%22parsedDate%22%3A%222016-06-15%22%2C%22numChildren%22%3A3%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EOrgil%2C%20O.%2C%20Mor%2C%20H.%2C%20Matityahu%2C%20A.%2C%20%26amp%3B%20Onn%2C%20I.%20%282016%29.%20Identification%20of%20a%20region%20in%20the%20coiled-coil%20domain%20of%20Smc3%20that%20is%20essential%20for%20cohesin%20activity.%20%3Ci%3ENucleic%20Acids%20Research%3C%5C%2Fi%3E%2C%20gkw539.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Fnar%5C%2Fgkw539%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Fnar%5C%2Fgkw539%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Identification%20of%20a%20region%20in%20the%20coiled-coil%20domain%20of%20Smc3%20that%20is%20essential%20for%20cohesin%20activity%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ola%22%2C%22lastName%22%3A%22Orgil%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hadar%22%2C%22lastName%22%3A%22Mor%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Avi%22%2C%22lastName%22%3A%22Matityahu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Itay%22%2C%22lastName%22%3A%22Onn%22%7D%5D%2C%22abstractNote%22%3A%22The%20cohesin%20complex%20plays%20an%20important%20role%20in%20sister%20chromatin%20cohesion.%20Cohesin%27s%20core%20is%20composed%20of%20two%20structural%20maintenance%20of%20chromosome%20%28SMC%29%20proteins%2C%20called%20Smc1%20and%20Smc3.%20SMC%20proteins%20are%20built%20from%20a%20globular%20hinge%20domain%2C%20a%20rod-shaped%20domain%20composed%20of%20long%20anti-parallel%20coiled-coil%20%28CC%29%2C%20and%20a%20second%20globular%20adenosine%20triphosphatase%20domain%20called%20the%20head.%20The%20functions%20of%20both%20head%20and%20hinge%20domains%20have%20been%20studied%20extensively%2C%20yet%20the%20function%20of%20the%20CC%20region%20remains%20elusive.%20We%20identified%20a%20mutation%20in%20the%20CC%20of%20smc3%20%28L217P%29%20that%20disrupts%20the%20function%20of%20the%20protein.%20Cells%20carrying%20the%20smc3-L217P%20allele%20have%20a%20strong%20cohesion%20defect%20and%20complexes%20containing%20smc3-L217P%20are%20not%20loaded%20onto%20the%20chromosomes.%20However%2C%20the%20mutation%20does%20not%20affect%20inter-protein%20interactions%20in%20either%20the%20core%20complex%20or%20with%20the%20Scc2%20loader.%20We%20show%20by%20molecular%20dynamics%20and%20biochemistry%20that%20wild-type%20Smc3%20can%20adopt%20distinct%20conformations%2C%20and%20that%20adenosine%20triphosphate%20%28ATP%29%20induces%20the%20conformational%20change.%20The%20L217P%20mutation%20restricts%20the%20ability%20of%20the%20mutated%20protein%20to%20switch%20between%20the%20conformations.%20We%20suggest%20that%20the%20function%20of%20the%20CC%20is%20to%20transfer%20ATP%20binding%5C%2Fhydrolysis%20signals%20between%20the%20head%20and%20the%20hinge%20domains.%20The%20results%20provide%20a%20new%20insight%20into%20the%20mechanism%20of%20cohesin%20activity.%22%2C%22date%22%3A%222016-06-15%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1093%5C%2Fnar%5C%2Fgkw539%22%2C%22ISSN%22%3A%220305-1048%2C%201362-4962%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fnar.oxfordjournals.org%5C%2Fcontent%5C%2Fearly%5C%2F2016%5C%2F06%5C%2F15%5C%2Fnar.gkw539%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-06-24T15%3A22%3A15Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Cultured%20cells%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-yeast%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%2C%7B%22tag%22%3A%22Yeast%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22VCUJTQ4J%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Shwartz%20et%20al.%22%2C%22parsedDate%22%3A%222016-06-08%22%2C%22numChildren%22%3A3%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EShwartz%2C%20M.%2C%20Matityahu%2C%20A.%2C%20%26amp%3B%20Onn%2C%20I.%20%282016%29.%20Identification%20of%20Functional%20Domains%20in%20the%20Cohesin%20Loader%20Subunit%20Scc4%20by%20a%20Random%20Insertion%5C%2FDominant%20Negative%20Screen.%20%3Ci%3EG3%3A%20Genes%7CGenomes%7CGenetics%3C%5C%2Fi%3E%2C%20g3.116.031674.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1534%5C%2Fg3.116.031674%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1534%5C%2Fg3.116.031674%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Identification%20of%20Functional%20Domains%20in%20the%20Cohesin%20Loader%20Subunit%20Scc4%20by%20a%20Random%20Insertion%5C%2FDominant%20Negative%20Screen%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michal%22%2C%22lastName%22%3A%22Shwartz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Avi%22%2C%22lastName%22%3A%22Matityahu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Itay%22%2C%22lastName%22%3A%22Onn%22%7D%5D%2C%22abstractNote%22%3A%22Cohesin%20is%20a%20multi-subunit%20complex%20that%20plays%20an%20essential%20role%20in%20genome%20stability.%20Initial%20association%20of%20cohesin%20with%20chromosomes%20requires%20the%20loader%2C%20a%20heterodimer%20composed%20of%20Scc4%20and%20Scc2.%20However%2C%20very%20little%20is%20known%20about%20the%20loader%27s%20mechanism%20of%20action.%20In%20this%20study%20we%20performed%20a%20genetic%20screen%20to%20identify%20functional%20domains%20in%20the%20Scc4%20subunit%20of%20the%20loader.%20We%20isolated%20scc4%20mutant%20alleles%20that%20when%20overexpressed%20have%20a%20dominant%20negative%20effect%20on%20cell%20viability.%20We%20defined%20a%20small%20region%20in%20the%20N%27%20terminal%20of%20Scc4%20that%20is%20dominant%20negative%20when%20overexpressed%2C%20and%20on%20which%20Scc2%5C%2F4%20activity%20depends.%20When%20the%20mutant%20alleles%20are%20expressed%20as%20a%20single%20copy%2C%20they%20are%20recessive%20and%20do%20not%20support%20cell%20viability%2C%20cohesion%2C%20cohesin%20loading%20or%20Scc4%20chromatin%20binding.%20In%20addition%2C%20we%20show%20that%20the%20mutants%20investigated%20reduce%20but%20do%20not%20eliminate%20the%20interaction%20of%20Scc4%20with%20either%20Scc2%20or%20cohesin.%20However%2C%20we%20show%20that%20Scc4%20cannot%20bind%20cohesin%20in%20the%20absence%20of%20Scc2.%20Our%20results%20provide%20new%20insight%20into%20the%20roles%20of%20Scc4%20in%20cohesin%20loading%20and%20contribute%20to%20the%20deciphering%20of%20the%20loading%20mechanism.%22%2C%22date%22%3A%222016-06-08%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1534%5C%2Fg3.116.031674%22%2C%22ISSN%22%3A%22%2C%202160-1836%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.g3journal.org%5C%2Fcontent%5C%2Fearly%5C%2F2016%5C%2F06%5C%2F06%5C%2Fg3.116.031674%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-06-24T16%3A10%3A35Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Cultured%20cells%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-yeast%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%2C%7B%22tag%22%3A%22Saccharomyces%20cerevisiae%22%7D%2C%7B%22tag%22%3A%22Yeast%22%7D%5D%7D%7D%2C%7B%22key%22%3A%222MADWFHW%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Zheng%20et%20al.%22%2C%22parsedDate%22%3A%222016-06%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EZheng%2C%20Y.%2C%20Xie%2C%20J.%2C%20Huang%2C%20X.%2C%20Dong%2C%20J.%2C%20Park%2C%20M.%20S.%2C%20%26amp%3B%20Chan%2C%20W.%20K.%20%282016%29.%20Binding%20studies%20using%20Pichia%20pastoris%20expressed%20human%20aryl%20hydrocarbon%20receptor%20and%20aryl%20hydrocarbon%20receptor%20nuclear%20translocator%20proteins.%20%3Ci%3EProtein%20Expression%20and%20Purification%3C%5C%2Fi%3E%2C%20%3Ci%3E122%3C%5C%2Fi%3E%2C%2072%26%23x2013%3B81.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.pep.2016.02.011%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.pep.2016.02.011%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Binding%20studies%20using%20Pichia%20pastoris%20expressed%20human%20aryl%20hydrocarbon%20receptor%20and%20aryl%20hydrocarbon%20receptor%20nuclear%20translocator%20proteins%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yujuan%22%2C%22lastName%22%3A%22Zheng%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jinghang%22%2C%22lastName%22%3A%22Xie%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Xin%22%2C%22lastName%22%3A%22Huang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jin%22%2C%22lastName%22%3A%22Dong%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Miki%20S.%22%2C%22lastName%22%3A%22Park%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22William%20K.%22%2C%22lastName%22%3A%22Chan%22%7D%5D%2C%22abstractNote%22%3A%22The%20aryl%20hydrocarbon%20receptor%20%28AHR%29%20is%20a%20transcription%20factor%20which%20activates%20gene%20transcription%20by%20binding%20to%20its%20corresponding%20enhancer%20as%20the%20heterodimer%2C%20which%20is%20consisted%20of%20AHR%20and%20the%20aryl%20hydrocarbon%20receptor%20nuclear%20translocator%20%28ARNT%29.%20Human%20AHR%20can%20be%20rather%20difficult%20to%20study%2C%20when%20compared%20among%20the%20AHR%20of%20other%20species%2C%20since%20it%20is%20relatively%20unstable%20and%20less%20sensitive%20to%20some%20ligands%20in%5Cu00a0vitro.%20Overexpression%20of%20human%20AHR%20has%20been%20limited%20to%20the%20baculovirus%20expression%2C%20which%20is%20costly%20and%20tedious%20due%20to%20the%20need%20of%20repetitive%20baculovirus%20production.%20Here%20we%20explored%20whether%20we%20could%20generate%20abundant%20amounts%20of%20human%20AHR%20and%20ARNT%20in%20a%20better%20overexpression%20system%20for%20functional%20study.%20We%20observed%20that%20human%20AHR%20and%20ARNT%20can%20be%20expressed%20in%20Pichia%20pastoris%20with%20yields%20that%20are%20comparable%20to%20the%20baculovirus%20system%20only%20if%20their%20cDNAs%20are%20optimized%20for%20Pichia%20expression.%20Fusion%20with%20a%20c-myc%20tag%20at%20their%20C-termini%20seems%20to%20increase%20the%20expression%20yield.%20These%20Pichia%20expressed%20proteins%20can%20effectively%20heterodimerize%20and%20form%20the%20ternary%20AHR%5C%2FARNT%5C%2Fenhancer%20complex%20in%20the%20presence%20of%20%5Cu03b2-naphthoflavone%20or%20kynurenine.%20Limited%20proteolysis%20using%20thermolysin%20can%20be%20used%20to%20study%20the%20heterodimerization%20of%20these%20human%20AHR%20and%20ARNT%20proteins.%22%2C%22date%22%3A%22June%202016%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.pep.2016.02.011%22%2C%22ISSN%22%3A%221046-5928%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fpii%5C%2FS1046592816300316%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-06-24T15%3A24%3A33Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Cell%20culture%22%7D%2C%7B%22tag%22%3A%22DNA%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-yeast%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Pichia%20pastoris%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%2C%7B%22tag%22%3A%22Yeast%22%7D%5D%7D%7D%2C%7B%22key%22%3A%224DI9RTGZ%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mashock%22%2C%22parsedDate%22%3A%222016-05%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EMashock%2C%20M.%20J.%20%282016%29.%20%3Ci%3EExamining%20mechanism%20of%20toxicity%20of%20copper%20oxide%20nanoparticles%20to%20Saccharomyces%20cerevisiae%20and%20Caenorhabditis%20elegans%3C%5C%2Fi%3E.%20Marquette%20University.%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22thesis%22%2C%22title%22%3A%22Examining%20mechanism%20of%20toxicity%20of%20copper%20oxide%20nanoparticles%20to%20Saccharomyces%20cerevisiae%20and%20Caenorhabditis%20elegans%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michael%20Joseph%22%2C%22lastName%22%3A%22Mashock%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22thesisType%22%3A%22%22%2C%22university%22%3A%22Marquette%20University%22%2C%22date%22%3A%22May%202016%22%2C%22language%22%3A%22%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-06-29T19%3A51%3A46Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Cultured%20cells%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-yeast%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22RNA%22%7D%2C%7B%22tag%22%3A%22Yeast%22%7D%5D%7D%7D%2C%7B%22key%22%3A%2263E7XRAQ%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Avbelj%20et%20al.%22%2C%22parsedDate%22%3A%222015-09-30%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EAvbelj%2C%20M.%2C%20Zupan%2C%20J.%2C%20Kranjc%2C%20L.%2C%20%26amp%3B%20Raspor%2C%20P.%20%282015%29.%20Quorum-Sensing%20Kinetics%20in%20%3Ci%3ESaccharomyces%20cerevisiae%3C%5C%2Fi%3E%26%23x202F%3B%3A%20A%20Symphony%20of%20%3Ci%3EARO%3C%5C%2Fi%3E%20Genes%20and%20Aromatic%20Alcohols.%20%3Ci%3EJournal%20of%20Agricultural%20and%20Food%20Chemistry%3C%5C%2Fi%3E%2C%20%3Ci%3E63%3C%5C%2Fi%3E%2838%29%2C%208544%26%23x2013%3B8550.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.jafc.5b03400%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.jafc.5b03400%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Quorum-Sensing%20Kinetics%20in%20%3Ci%3ESaccharomyces%20cerevisiae%3C%5C%2Fi%3E%20%3A%20A%20Symphony%20of%20%3Ci%3EARO%3C%5C%2Fi%3E%20Genes%20and%20Aromatic%20Alcohols%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Martina%22%2C%22lastName%22%3A%22Avbelj%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jure%22%2C%22lastName%22%3A%22Zupan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Luka%22%2C%22lastName%22%3A%22Kranjc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Peter%22%2C%22lastName%22%3A%22Raspor%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222015-09-30%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1021%5C%2Facs.jafc.5b03400%22%2C%22ISSN%22%3A%220021-8561%2C%201520-5118%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fpubs.acs.org%5C%2Fdoi%5C%2F10.1021%5C%2Facs.jafc.5b03400%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-10-30T20%3A17%3A01Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Cultured%20cells%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-yeast%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22RNA%22%7D%2C%7B%22tag%22%3A%22Saccharomyces%20cerevisiae%22%7D%2C%7B%22tag%22%3A%22Yeast%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22GTXA48W3%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Meyer%20et%20al.%22%2C%22parsedDate%22%3A%222015-09-01%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EMeyer%2C%20R.%20E.%2C%20Chuong%2C%20H.%20H.%2C%20Hild%2C%20M.%2C%20Hansen%2C%20C.%20L.%2C%20Kinter%2C%20M.%2C%20%26amp%3B%20Dawson%2C%20D.%20S.%20%282015%29.%20Ipl1%5C%2FAurora-B%20is%20necessary%20for%20kinetochore%20restructuring%20in%20meiosis%20I%20in%20Saccharomyces%20cerevisiae.%20%3Ci%3EMolecular%20Biology%20of%20the%20Cell%3C%5C%2Fi%3E%2C%20%3Ci%3E26%3C%5C%2Fi%3E%2817%29%2C%202986%26%23x2013%3B3000.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1091%5C%2Fmbc.E15-01-0032%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1091%5C%2Fmbc.E15-01-0032%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Ipl1%5C%2FAurora-B%20is%20necessary%20for%20kinetochore%20restructuring%20in%20meiosis%20I%20in%20Saccharomyces%20cerevisiae%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20E.%22%2C%22lastName%22%3A%22Meyer%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22H.%20H.%22%2C%22lastName%22%3A%22Chuong%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Hild%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%20L.%22%2C%22lastName%22%3A%22Hansen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Kinter%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22D.%20S.%22%2C%22lastName%22%3A%22Dawson%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222015-09-01%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1091%5C%2Fmbc.E15-01-0032%22%2C%22ISSN%22%3A%221059-1524%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.molbiolcell.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1091%5C%2Fmbc.E15-01-0032%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-12-31T21%3A13%3A17Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Cell%20culture%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-yeast%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%2C%7B%22tag%22%3A%22Tissue%20culture%22%7D%2C%7B%22tag%22%3A%22Yeast%22%7D%5D%7D%7D%2C%7B%22key%22%3A%226XP87W28%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Aylward%20et%20al.%22%2C%22parsedDate%22%3A%222015-08-28%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EAylward%2C%20F.%20O.%2C%20Khadempour%2C%20L.%2C%20Tremmel%2C%20D.%20M.%2C%20McDonald%2C%20B.%20R.%2C%20Nicora%2C%20C.%20D.%2C%20Wu%2C%20S.%2C%20Moore%2C%20R.%20J.%2C%20Orton%2C%20D.%20J.%2C%20Monroe%2C%20M.%20E.%2C%20Piehowski%2C%20P.%20D.%2C%20Purvine%2C%20S.%20O.%2C%20Smith%2C%20R.%20D.%2C%20Lipton%2C%20M.%20S.%2C%20Burnum-Johnson%2C%20K.%20E.%2C%20%26amp%3B%20Currie%2C%20C.%20R.%20%282015%29.%20Enrichment%20and%20Broad%20Representation%20of%20Plant%20Biomass-Degrading%20Enzymes%20in%20the%20Specialized%20Hyphal%20Swellings%20of%20Leucoagaricus%20gongylophorus%2C%20the%20Fungal%20Symbiont%20of%20Leaf-Cutter%20Ants.%20%3Ci%3EPLOS%20ONE%3C%5C%2Fi%3E%2C%20%3Ci%3E10%3C%5C%2Fi%3E%288%29%2C%20e0134752.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0134752%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0134752%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Enrichment%20and%20Broad%20Representation%20of%20Plant%20Biomass-Degrading%20Enzymes%20in%20the%20Specialized%20Hyphal%20Swellings%20of%20Leucoagaricus%20gongylophorus%2C%20the%20Fungal%20Symbiont%20of%20Leaf-Cutter%20Ants%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Frank%20O.%22%2C%22lastName%22%3A%22Aylward%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lily%22%2C%22lastName%22%3A%22Khadempour%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daniel%20M.%22%2C%22lastName%22%3A%22Tremmel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bradon%20R.%22%2C%22lastName%22%3A%22McDonald%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Carrie%20D.%22%2C%22lastName%22%3A%22Nicora%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Si%22%2C%22lastName%22%3A%22Wu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ronald%20J.%22%2C%22lastName%22%3A%22Moore%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daniel%20J.%22%2C%22lastName%22%3A%22Orton%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matthew%20E.%22%2C%22lastName%22%3A%22Monroe%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Paul%20D.%22%2C%22lastName%22%3A%22Piehowski%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Samuel%20O.%22%2C%22lastName%22%3A%22Purvine%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Richard%20D.%22%2C%22lastName%22%3A%22Smith%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mary%20S.%22%2C%22lastName%22%3A%22Lipton%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kristin%20E.%22%2C%22lastName%22%3A%22Burnum-Johnson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cameron%20R.%22%2C%22lastName%22%3A%22Currie%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Sean%22%2C%22lastName%22%3A%22Brady%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222015-8-28%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.pone.0134752%22%2C%22ISSN%22%3A%221932-6203%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.plos.org%5C%2F10.1371%5C%2Fjournal.pone.0134752%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-10-29T21%3A45%3A36Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-other%22%7D%2C%7B%22tag%22%3A%22Lipids%22%7D%2C%7B%22tag%22%3A%22Mushroom%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%2C%7B%22tag%22%3A%22Small%20molecules%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22QA9T8G2K%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Desai%20et%20al.%22%2C%22parsedDate%22%3A%222015-07-24%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EDesai%2C%20J.%2C%20Cheng%2C%20S.%2C%20Ying%2C%20T.%2C%20Nguyen%2C%20M.%2C%20Clancy%2C%20C.%2C%20Lanni%2C%20F.%2C%20%26amp%3B%20Mitchell%2C%20A.%20%282015%29.%20Coordination%20of%20Candida%20albicans%20Invasion%20and%20Infection%20Functions%20by%20Phosphoglycerol%20Phosphatase%20Rhr2.%20%3Ci%3EPathogens%3C%5C%2Fi%3E%2C%20%3Ci%3E4%3C%5C%2Fi%3E%283%29%2C%20573%26%23x2013%3B589.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fpathogens4030573%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fpathogens4030573%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Coordination%20of%20Candida%20albicans%20Invasion%20and%20Infection%20Functions%20by%20Phosphoglycerol%20Phosphatase%20Rhr2%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jigar%22%2C%22lastName%22%3A%22Desai%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shaoji%22%2C%22lastName%22%3A%22Cheng%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tammy%22%2C%22lastName%22%3A%22Ying%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Nguyen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cornelius%22%2C%22lastName%22%3A%22Clancy%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Frederick%22%2C%22lastName%22%3A%22Lanni%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aaron%22%2C%22lastName%22%3A%22Mitchell%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222015-07-24%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.3390%5C%2Fpathogens4030573%22%2C%22ISSN%22%3A%222076-0817%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F2076-0817%5C%2F4%5C%2F3%5C%2F573%5C%2F%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-12-31T21%3A41%3A55Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Candida%20albicans%22%7D%2C%7B%22tag%22%3A%22Cell%20culture%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-yeast%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22RNA%22%7D%2C%7B%22tag%22%3A%22Yeast%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22RDBF8WQ4%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Zhang%20et%20al.%22%2C%22parsedDate%22%3A%222015-03-31%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EZhang%2C%20L.%2C%20Li%2C%20X.%2C%20Hill%2C%20R.%20C.%2C%20Qiu%2C%20Y.%2C%20Zhang%2C%20W.%2C%20Hansen%2C%20K.%20C.%2C%20%26amp%3B%20Zhao%2C%20R.%20%282015%29.%20Brr2%20plays%20a%20role%20in%20spliceosomal%20activation%20in%20addition%20to%20U4%5C%2FU6%20unwinding.%20%3Ci%3ENucleic%20Acids%20Research%3C%5C%2Fi%3E%2C%20%3Ci%3E43%3C%5C%2Fi%3E%286%29%2C%203286%26%23x2013%3B3297.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Fnar%5C%2Fgkv062%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Fnar%5C%2Fgkv062%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Brr2%20plays%20a%20role%20in%20spliceosomal%20activation%20in%20addition%20to%20U4%5C%2FU6%20unwinding%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22L.%22%2C%22lastName%22%3A%22Zhang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22X.%22%2C%22lastName%22%3A%22Li%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20C.%22%2C%22lastName%22%3A%22Hill%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Y.%22%2C%22lastName%22%3A%22Qiu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22W.%22%2C%22lastName%22%3A%22Zhang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22K.%20C.%22%2C%22lastName%22%3A%22Hansen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%22%2C%22lastName%22%3A%22Zhao%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222015-03-31%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1093%5C%2Fnar%5C%2Fgkv062%22%2C%22ISSN%22%3A%220305-1048%2C%201362-4962%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fnar.oxfordjournals.org%5C%2Flookup%5C%2Fdoi%5C%2F10.1093%5C%2Fnar%5C%2Fgkv062%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-12-31T19%3A44%3A28Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Cell%20culture%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-yeast%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22RNA%22%7D%2C%7B%22tag%22%3A%22Yeast%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22AD3QC58U%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Orgil%20et%20al.%22%2C%22parsedDate%22%3A%222015-03-06%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EOrgil%2C%20O.%2C%20Matityahu%2C%20A.%2C%20Eng%2C%20T.%2C%20Guacci%2C%20V.%2C%20Koshland%2C%20D.%2C%20%26amp%3B%20Onn%2C%20I.%20%282015%29.%20A%20Conserved%20Domain%20in%20the%20Scc3%20Subunit%20of%20Cohesin%20Mediates%20the%20Interaction%20with%20Both%20Mcd1%20and%20the%20Cohesin%20Loader%20Complex.%20%3Ci%3EPLOS%20Genetics%3C%5C%2Fi%3E%2C%20%3Ci%3E11%3C%5C%2Fi%3E%283%29%2C%20e1005036.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pgen.1005036%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pgen.1005036%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20Conserved%20Domain%20in%20the%20Scc3%20Subunit%20of%20Cohesin%20Mediates%20the%20Interaction%20with%20Both%20Mcd1%20and%20the%20Cohesin%20Loader%20Complex%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ola%22%2C%22lastName%22%3A%22Orgil%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Avi%22%2C%22lastName%22%3A%22Matityahu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Eng%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Vincent%22%2C%22lastName%22%3A%22Guacci%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Douglas%22%2C%22lastName%22%3A%22Koshland%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Itay%22%2C%22lastName%22%3A%22Onn%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Rolf%22%2C%22lastName%22%3A%22Jessberger%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222015-3-6%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.pgen.1005036%22%2C%22ISSN%22%3A%221553-7404%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.plos.org%5C%2F10.1371%5C%2Fjournal.pgen.1005036%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-01-04T21%3A44%3A28Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Cultured%20cells%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-yeast%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%2C%7B%22tag%22%3A%22Yeast%22%7D%5D%7D%7D%2C%7B%22key%22%3A%224GZ5KIBG%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kurtzman%20and%20Robnett%22%2C%22parsedDate%22%3A%222014%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EKurtzman%2C%20C.%20P.%2C%20%26amp%3B%20Robnett%2C%20C.%20J.%20%282014%29.%20Three%20new%20anascosporic%20genera%20of%20the%20Saccharomycotina%3A%20Danielozyma%20gen.%20nov.%2C%20Deakozyma%20gen.%20nov.%20and%20Middelhovenomyces%20gen.%20nov.%20%3Ci%3EAntonie%20van%20Leeuwenhoek%3C%5C%2Fi%3E%2C%20%3Ci%3E105%3C%5C%2Fi%3E%285%29%2C%20933%26%23x2013%3B942.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs10482-014-0149-9%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs10482-014-0149-9%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Three%20new%20anascosporic%20genera%20of%20the%20Saccharomycotina%3A%20Danielozyma%20gen.%20nov.%2C%20Deakozyma%20gen.%20nov.%20and%20Middelhovenomyces%20gen.%20nov%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cletus%20P.%22%2C%22lastName%22%3A%22Kurtzman%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christie%20J.%22%2C%22lastName%22%3A%22Robnett%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%225%5C%2F2014%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1007%5C%2Fs10482-014-0149-9%22%2C%22ISSN%22%3A%220003-6072%2C%201572-9699%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flink.springer.com%5C%2F10.1007%5C%2Fs10482-014-0149-9%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-08-03T15%3A37%3A40Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Cultured%20cells%22%7D%2C%7B%22tag%22%3A%22DNA%22%7D%2C%7B%22tag%22%3A%22Danielozyma%22%7D%2C%7B%22tag%22%3A%22Deakozyma%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-yeast%22%7D%2C%7B%22tag%22%3A%22Middelhovenomyces%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Saccharomycotina%22%7D%2C%7B%22tag%22%3A%22Yeast%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22WIJK22KQ%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Wiedner%20et%20al.%22%2C%22parsedDate%22%3A%222013-07-01%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EWiedner%2C%20S.%20D.%2C%20Ansong%2C%20C.%2C%20Webb-Robertson%2C%20B.-J.%2C%20Pederson%2C%20L.%20M.%2C%20Fortuin%2C%20S.%2C%20Hofstad%2C%20B.%20A.%2C%20Shukla%2C%20A.%20K.%2C%20Panisko%2C%20E.%20A.%2C%20Smith%2C%20R.%20D.%2C%20%26amp%3B%20Wright%2C%20A.%20T.%20%282013%29.%20Disparate%20Proteome%20Responses%20of%20Pathogenic%20and%20Nonpathogenic%20Aspergilli%20to%20Human%20Serum%20Measured%20by%20Activity-Based%20Protein%20Profiling%20%28ABPP%29.%20%3Ci%3EMolecular%20%26amp%3B%20Cellular%20Proteomics%3C%5C%2Fi%3E%2C%20%3Ci%3E12%3C%5C%2Fi%3E%287%29%2C%201791%26%23x2013%3B1805.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1074%5C%2Fmcp.M112.026534%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1074%5C%2Fmcp.M112.026534%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Disparate%20Proteome%20Responses%20of%20Pathogenic%20and%20Nonpathogenic%20Aspergilli%20to%20Human%20Serum%20Measured%20by%20Activity-Based%20Protein%20Profiling%20%28ABPP%29%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%20D.%22%2C%22lastName%22%3A%22Wiedner%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%22%2C%22lastName%22%3A%22Ansong%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22B.-J.%22%2C%22lastName%22%3A%22Webb-Robertson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22L.%20M.%22%2C%22lastName%22%3A%22Pederson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%22%2C%22lastName%22%3A%22Fortuin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22B.%20A.%22%2C%22lastName%22%3A%22Hofstad%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%20K.%22%2C%22lastName%22%3A%22Shukla%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22E.%20A.%22%2C%22lastName%22%3A%22Panisko%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20D.%22%2C%22lastName%22%3A%22Smith%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%20T.%22%2C%22lastName%22%3A%22Wright%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222013-07-01%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1074%5C%2Fmcp.M112.026534%22%2C%22ISSN%22%3A%221535-9476%2C%201535-9484%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.mcponline.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1074%5C%2Fmcp.M112.026534%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T15%3A18%3A11Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Aspergillus%20clavatus%22%7D%2C%7B%22tag%22%3A%22Aspergillus%20fumigatus%22%7D%2C%7B%22tag%22%3A%22Cell%20Suspension%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-other%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Neosartorya%20fischeri%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22B7JEKX55%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Wiedner%20et%20al.%22%2C%22parsedDate%22%3A%222012-09-28%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EWiedner%2C%20S.%20D.%2C%20Burnum%2C%20K.%20E.%2C%20Pederson%2C%20L.%20M.%2C%20Anderson%2C%20L.%20N.%2C%20Fortuin%2C%20S.%2C%20Chauvigne-Hines%2C%20L.%20M.%2C%20Shukla%2C%20A.%20K.%2C%20Ansong%2C%20C.%2C%20Panisko%2C%20E.%20A.%2C%20Smith%2C%20R.%20D.%2C%20%26amp%3B%20Wright%2C%20A.%20T.%20%282012%29.%20Multiplexed%20Activity-based%20Protein%20Profiling%20of%20the%20Human%20Pathogen%20Aspergillus%20fumigatus%20Reveals%20Large%20Functional%20Changes%20upon%20Exposure%20to%20Human%20Serum.%20%3Ci%3EJournal%20of%20Biological%20Chemistry%3C%5C%2Fi%3E%2C%20%3Ci%3E287%3C%5C%2Fi%3E%2840%29%2C%2033447%26%23x2013%3B33459.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1074%5C%2Fjbc.M112.394106%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1074%5C%2Fjbc.M112.394106%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Multiplexed%20Activity-based%20Protein%20Profiling%20of%20the%20Human%20Pathogen%20Aspergillus%20fumigatus%20Reveals%20Large%20Functional%20Changes%20upon%20Exposure%20to%20Human%20Serum%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%20D.%22%2C%22lastName%22%3A%22Wiedner%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22K.%20E.%22%2C%22lastName%22%3A%22Burnum%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22L.%20M.%22%2C%22lastName%22%3A%22Pederson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22L.%20N.%22%2C%22lastName%22%3A%22Anderson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%22%2C%22lastName%22%3A%22Fortuin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22L.%20M.%22%2C%22lastName%22%3A%22Chauvigne-Hines%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%20K.%22%2C%22lastName%22%3A%22Shukla%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%22%2C%22lastName%22%3A%22Ansong%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22E.%20A.%22%2C%22lastName%22%3A%22Panisko%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20D.%22%2C%22lastName%22%3A%22Smith%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%20T.%22%2C%22lastName%22%3A%22Wright%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222012-09-28%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1074%5C%2Fjbc.M112.394106%22%2C%22ISSN%22%3A%220021-9258%2C%201083-351X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.jbc.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1074%5C%2Fjbc.M112.394106%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T14%3A58%3A29Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Aspergillus%20fumigatus%22%7D%2C%7B%22tag%22%3A%22Cultured%20cells%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-other%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Proteins%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22WVQDAF73%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Yin%20et%20al.%22%2C%22parsedDate%22%3A%222012%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EYin%2C%20Y.%2C%20Liu%2C%20Y.%2C%20Jin%2C%20H.%2C%20Wang%2C%20S.%2C%20Zhao%2C%20S.%2C%20Geng%2C%20X.%2C%20Li%2C%20M.%2C%20%26amp%3B%20Xu%2C%20F.%20%282012%29.%20Polyethylene%20glycol-mediated%20transformation%20of%20fused%20egfp-hph%20gene%20under%20the%20control%20of%20gpd%20promoter%20in%20Pleurotus%20eryngii.%20%3Ci%3EBiotechnology%20Letters%3C%5C%2Fi%3E%2C%20%3Ci%3E34%3C%5C%2Fi%3E%2810%29%2C%201895%26%23x2013%3B1900.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs10529-012-0985-5%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs10529-012-0985-5%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Polyethylene%20glycol-mediated%20transformation%20of%20fused%20egfp-hph%20gene%20under%20the%20control%20of%20gpd%20promoter%20in%20Pleurotus%20eryngii%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yonggang%22%2C%22lastName%22%3A%22Yin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yu%22%2C%22lastName%22%3A%22Liu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Haojie%22%2C%22lastName%22%3A%22Jin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shouxian%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shuang%22%2C%22lastName%22%3A%22Zhao%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Xiaoli%22%2C%22lastName%22%3A%22Geng%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ming%22%2C%22lastName%22%3A%22Li%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Feng%22%2C%22lastName%22%3A%22Xu%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2210%5C%2F2012%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1007%5C%2Fs10529-012-0985-5%22%2C%22ISSN%22%3A%220141-5492%2C%201573-6776%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flink.springer.com%5C%2F10.1007%5C%2Fs10529-012-0985-5%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T15%3A14%3A14Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22DNA%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Fungi-other%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%2C%7B%22tag%22%3A%22Pleurotus%20eryngii%22%7D%5D%7D%7D%2C%7B%22key%22%3A%22M3GMSWHC%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Suzuki%20et%20al.%22%2C%22parsedDate%22%3A%222012%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ESuzuki%2C%20Y.%2C%20Murray%2C%20S.%20L.%2C%20Wong%2C%20K.%20H.%2C%20Davis%2C%20M.%20A.%2C%20%26amp%3B%20Hynes%2C%20M.%20J.%20%282012%29.%20Reprogramming%20of%20carbon%20metabolism%20by%20the%20transcriptional%20activators%20AcuK%20and%20AcuM%20in%20Aspergillus%20nidulans%3A%20Transcription%20factors%20controlling%20carbon%20metabolism.%20%3Ci%3EMolecular%20Microbiology%3C%5C%2Fi%3E%2C%20%3Ci%3E84%3C%5C%2Fi%3E%285%29%2C%20942%26%23x2013%3B964.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2Fj.1365-2958.2012.08067.x%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2Fj.1365-2958.2012.08067.x%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Reprogramming%20of%20carbon%20metabolism%20by%20the%20transcriptional%20activators%20AcuK%20and%20AcuM%20in%20Aspergillus%20nidulans%3A%20Transcription%20factors%20controlling%20carbon%20metabolism%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yumi%22%2C%22lastName%22%3A%22Suzuki%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sandra%20L.%22%2C%22lastName%22%3A%22Murray%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Koon%20Ho%22%2C%22lastName%22%3A%22Wong%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Meryl%20A.%22%2C%22lastName%22%3A%22Davis%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michael%20J.%22%2C%22lastName%22%3A%22Hynes%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2206%5C%2F2012%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1111%5C%2Fj.1365-2958.2012.08067.x%22%2C%22ISSN%22%3A%220950382X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdoi.wiley.com%5C%2F10.1111%5C%2Fj.1365-2958.2012.08067.x%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-09T15%3A06%3A47Z%22%2C%22tags%22%3A%5B%7B%22tag%22%3A%22Aspergillus%20nidulans%22%7D%2C%7B%22tag%22%3A%22DNA%22%7D%2C%7B%22tag%22%3A%22Fungi%22%7D%2C%7B%22tag%22%3A%22Molecules%22%7D%2C%7B%22tag%22%3A%22Plants-algae-fungi%22%7D%5D%7D%7D%5D%7D
Rasool, A., Ahmed, M. S., & Li, C. (2016). Overproduction of squalene synergistically downregulates ethanol production in Saccharomyces cerevisiae. Chemical Engineering Science, 152, 370–380. https://doi.org/10.1016/j.ces.2016.06.014
Orgil, O., Mor, H., Matityahu, A., & Onn, I. (2016). Identification of a region in the coiled-coil domain of Smc3 that is essential for cohesin activity. Nucleic Acids Research, gkw539. https://doi.org/10.1093/nar/gkw539
Shwartz, M., Matityahu, A., & Onn, I. (2016). Identification of Functional Domains in the Cohesin Loader Subunit Scc4 by a Random Insertion/Dominant Negative Screen. G3: Genes|Genomes|Genetics, g3.116.031674. https://doi.org/10.1534/g3.116.031674
Zheng, Y., Xie, J., Huang, X., Dong, J., Park, M. S., & Chan, W. K. (2016). Binding studies using Pichia pastoris expressed human aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator proteins. Protein Expression and Purification, 122, 72–81. https://doi.org/10.1016/j.pep.2016.02.011
Mashock, M. J. (2016). Examining mechanism of toxicity of copper oxide nanoparticles to Saccharomyces cerevisiae and Caenorhabditis elegans. Marquette University.
Avbelj, M., Zupan, J., Kranjc, L., & Raspor, P. (2015). Quorum-Sensing Kinetics in Saccharomyces cerevisiae : A Symphony of ARO Genes and Aromatic Alcohols. Journal of Agricultural and Food Chemistry, 63(38), 8544–8550. https://doi.org/10.1021/acs.jafc.5b03400
Meyer, R. E., Chuong, H. H., Hild, M., Hansen, C. L., Kinter, M., & Dawson, D. S. (2015). Ipl1/Aurora-B is necessary for kinetochore restructuring in meiosis I in Saccharomyces cerevisiae. Molecular Biology of the Cell, 26(17), 2986–3000. https://doi.org/10.1091/mbc.E15-01-0032
Aylward, F. O., Khadempour, L., Tremmel, D. M., McDonald, B. R., Nicora, C. D., Wu, S., Moore, R. J., Orton, D. J., Monroe, M. E., Piehowski, P. D., Purvine, S. O., Smith, R. D., Lipton, M. S., Burnum-Johnson, K. E., & Currie, C. R. (2015). Enrichment and Broad Representation of Plant Biomass-Degrading Enzymes in the Specialized Hyphal Swellings of Leucoagaricus gongylophorus, the Fungal Symbiont of Leaf-Cutter Ants. PLOS ONE, 10(8), e0134752. https://doi.org/10.1371/journal.pone.0134752
Desai, J., Cheng, S., Ying, T., Nguyen, M., Clancy, C., Lanni, F., & Mitchell, A. (2015). Coordination of Candida albicans Invasion and Infection Functions by Phosphoglycerol Phosphatase Rhr2. Pathogens, 4(3), 573–589. https://doi.org/10.3390/pathogens4030573
Zhang, L., Li, X., Hill, R. C., Qiu, Y., Zhang, W., Hansen, K. C., & Zhao, R. (2015). Brr2 plays a role in spliceosomal activation in addition to U4/U6 unwinding. Nucleic Acids Research, 43(6), 3286–3297. https://doi.org/10.1093/nar/gkv062
Orgil, O., Matityahu, A., Eng, T., Guacci, V., Koshland, D., & Onn, I. (2015). A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex. PLOS Genetics, 11(3), e1005036. https://doi.org/10.1371/journal.pgen.1005036
Kurtzman, C. P., & Robnett, C. J. (2014). Three new anascosporic genera of the Saccharomycotina: Danielozyma gen. nov., Deakozyma gen. nov. and Middelhovenomyces gen. nov. Antonie van Leeuwenhoek, 105(5), 933–942. https://doi.org/10.1007/s10482-014-0149-9
Wiedner, S. D., Ansong, C., Webb-Robertson, B.-J., Pederson, L. M., Fortuin, S., Hofstad, B. A., Shukla, A. K., Panisko, E. A., Smith, R. D., & Wright, A. T. (2013). Disparate Proteome Responses of Pathogenic and Nonpathogenic Aspergilli to Human Serum Measured by Activity-Based Protein Profiling (ABPP). Molecular & Cellular Proteomics, 12(7), 1791–1805. https://doi.org/10.1074/mcp.M112.026534
Wiedner, S. D., Burnum, K. E., Pederson, L. M., Anderson, L. N., Fortuin, S., Chauvigne-Hines, L. M., Shukla, A. K., Ansong, C., Panisko, E. A., Smith, R. D., & Wright, A. T. (2012). Multiplexed Activity-based Protein Profiling of the Human Pathogen Aspergillus fumigatus Reveals Large Functional Changes upon Exposure to Human Serum. Journal of Biological Chemistry, 287(40), 33447–33459. https://doi.org/10.1074/jbc.M112.394106
Yin, Y., Liu, Y., Jin, H., Wang, S., Zhao, S., Geng, X., Li, M., & Xu, F. (2012). Polyethylene glycol-mediated transformation of fused egfp-hph gene under the control of gpd promoter in Pleurotus eryngii. Biotechnology Letters, 34(10), 1895–1900. https://doi.org/10.1007/s10529-012-0985-5
Suzuki, Y., Murray, S. L., Wong, K. H., Davis, M. A., & Hynes, M. J. (2012). Reprogramming of carbon metabolism by the transcriptional activators AcuK and AcuM in Aspergillus nidulans: Transcription factors controlling carbon metabolism. Molecular Microbiology, 84(5), 942–964. https://doi.org/10.1111/j.1365-2958.2012.08067.x