In order to ensure your success in using our products, please read the guidelines provided here. We did the homework so you don’t have to!
For more handy hints, check out our Homogenization Guide (with pictures).
Bullet Blender® Best Practices
Use high quality polypropylene tubes. Cheaper tubes may crack or leak through the cap.
Don’t use Parafilm to seal tubes. The tubes in a Bullet Blender need to be able to move freely. Parafilm can make the tubes jam in the holes, causing poor homogenization, tube damage and sample loss. If you need more leak resistance, consider using our RINO® tubes.
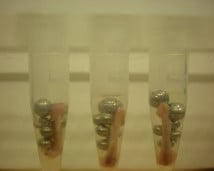
Sample preparation is important. Samples cut into thin strips will homogenize better than cube-shaped or round samples.
Choose your beads based on the toughness of the tissue. Visit the Bead Overview Page for recommendations.
Amounts of beads and buffer should be relative to the amount of tissue. The best proportions are usually 1 volume of beads, 1 volume of sample, and 2 volumes of buffer. For most tissues, 100 mg ≅ 100 μL. Example: To homogenize a 250-mg tissue sample, use 250 μL of beads and 500 μL of buffer.
IMPORTANT NOTE: If your protocol or kit calls for a larger volume of extraction reagent, use the volume that we recommend and add the remaining volume of reagent post-homogenization. You can then run the samples in the Bullet Blender for one minute at speed 2 to mix the tube contents. Adding too large a volume of homogenization buffer will prevent your sample from interacting vigorously with the beads.
Do not overload OR underload tubes. Using too much or too little material in the tube can prevent the sample from homogenizing fully. Use the table below for guidance.
It is important to match the contact plate installed in the instrument to the tubes being used. Using the incorrect contact plate may result in poor homogenization or prevent the cover from fully closing. For more information, visit the Contact Plates page.
| Model | Minimum Sample | Maximum Sample | Maximum Volume |
|---|---|---|---|
| Microcentrifuge models | 0.01g | 0.3g | 1.2 mL |
| 5 mL models | 0.1g | 1.0g | 3.5 mL |
| 50 mL models | 0.1g | 3.5g | 20 mL |
If one run is not satisfactory: Simply run the samples again! There is no need to change the beads or tubes.
Special Considerations for RNA isolation: Be sure to use RNase-free beads and tubes. Tubes and beads may be purchased RNase-free, or you may clean them yourself.
Phenol-based extraction reagents (TRIzol®, TRI Reagent®) are not recommended for use with stainless steel beads. We recommend using another type of bead with these reagents, or use a non-phenol based extraction reagent as the homogenization buffer, such as a guanidine thiocyanate based reagent.
Special Note for the Bullet Blender 50-DX: Load at least two tubes inside of sleeves in the Bullet Blender 50-DX. Due to the larger motor, tubes may break if the unit is underloaded. Water-filled tubes may be used for this purpose. Also, if you run the same tubes multiple times, be sure that the caps are tightly screwed on before each additional run.